by Tianna DuPont, Associate Professor, Washington State University; Tim Smith, Washington State University Tree Fruit Extension Specialist Emeritus; Ken Johnson, Professor of Plant Pathology Oregon State University; Youfu Zhao, Washington State University. Updated December 2024 peer reviewed publication FS391E. FS391E Fire Blight of Apple and Pear in Washington
Fire blight is an important disease affecting pear and apple. Infections commonly occur during bloom or on late blooms during the three weeks following petal fall. Increased acreage of highly susceptible apple varieties on highly susceptible rootstocks has increased the danger that infected blocks will suffer significant damage. In Washington there have been minor outbreaks annually since 1991 and serious damage in over 5% to 10% of orchards in 1993, 1997, 1998, 2005, 2009, 2012, 2015, 2016, 2017, and 2018. Controlling fire blight is a significant expense for pome fruit producers. For example, in epidemic outbreaks in Washington in 2017 and 2018 growers reported spending an average of $250 an acre on preventative sprays and $27 to $864 per acre for removal of cankered wood as well as removal of more than 300 acres of apples and 200 acres of pears.
Casual Organism
Fire blight is caused by Erwinia amylovora, a gram-negative, rod-shaped bacterium. The bacterial population grows via cell division, and the rate of division is regulated by temperature, among other factors. Cell division is minimal below 50°F and relatively slow at air temperatures between 50°F and 70°F. At air temperatures above 70°F, the rate of cell division increases rapidly and is fastest (optimal) at 80°F. Above 95°F cell density on and in the plant can actually decline (Pusey and Curry 2004).
Host Range
Considered a problem for apple and pear, E. amylovora has a wide host range infecting about 200 rosaceous plant species (Momol and Aldwincklke 2000). Most strains are able to infect commercial, ornamental, and wild plants from the subfamily Amygdaloideae, which includes apple, pear, quince, crab apple, hawthorn, mountain ash, and Bradford pear. However, some E. amylovora strains exclusively infect plants of the genus Rubus (Rosoideae subfamily), like blackberries and raspberries (Momol and Aldwincklke 2000; Bluhm and Stover 2016).
Signs and Symptoms
Overwintering cankers
Overwintering cankers can appear black, grey, violet, or brown (Figure 1). Older cankers may have dry, sunken tissue. If the bark is cut from the edge of an active canker, reddish flecking can be seen in the wood near the canker margin (Teviotdale 2011).
Blossom symptoms
Blossom symptoms become apparent one to two weeks after infection. The floral receptacle, ovary, and peduncles become water-soaked and dull, grayish green in appearance (Figure 2). Later tissues shrivel and turn brown to black. During periods of high humidity, small droplets of bacterial ooze form on water-soaked and discolored tissues. Ooze droplets appear creamy white when new and become amber tinted as they age (Johnson 2000).
Shoot symptoms
Shoot symptoms include the characteristic “shepherd’s crook” formed from rapidly wilting shoot tips. Leaves on diseased shoots often show blackening along the midrib and veins before becoming fully necrotic and will cling firmly to the host after death (a key diagnostic feature) (Figure 3). Numerous diseased shoots give a tree a burnt, blighted appearance (hence the disease name).
Rootstock infections
Rootstock infections usually develop near the graft union as a result of internal movement of the pathogen through the tree or from infection of root suckers. The bark of infected rootstocks may show water-soaking, purplish to black discoloration, cracking, or signs of bacterial ooze. Red-brown streaking may be apparent in the cambium just under the bark (Figure 4). Symptoms of rootstock blight can be confused with Phytophthora collar rot. Malling 26 and 9 rootstocks are highly susceptible to fire blight (Johnson 2000).
Transmission and Disease Cycle
Erwinia amylovora survives winter in the living tissue around canker margins and underneath the dark canker surface (Biggs 1994; Teviotdale 2011; Santander et al. 2022a). Live cells can survive the winter in 7% to 62% of cankers (van der Zwet and Beer 1991; Santander et al. 2022b), and as few as one canker in a block can cause a new infection (Tullis 1929). When humidity is high in the spring the pathogen emerges from cankers as ooze and oozeless colonies (Figure 5). This ooze is attractive to bees, flies, and other insects who transfer the pathogen to flowers (Van Der Zwet and Keil 1979). Pathogen cells can also be moved from old cankers to flowers and other susceptible tissues by water splash and wind-blown rain. Pathogen cells multiply quickly on nutrient rich floral stigma when temperatures are warm (70°F to 80°F is optimal for the pathogen) (Ogawa and English 1991; Pusey and Curry 2004). Bacterial cells can then be washed down the style into the floral cup by water (usually from rain or heavy dew) where they can invade flowers through the nectaries (Thomson 1986). Once initial blossoms are infested, ooze droplets form with as many as a billion bacterial cells per droplet (Slack et al. 2017). Insects and rain can move the pathogen to additional flowers and susceptible young leaves (Johnson et al. 1993; Pattemore et al. 2014). If the pathogen is successful in infecting the developing fruitlet, the disease spreads through the intercellular spaces and then the vascular system of the tree (xylem) (Momol et al. 1998), and more ooze can form on flower and fruitlet surfaces. The pathogen kills young host tissues as it progresses, creating characteristic lesions often called a strike. After invading woody tissues, it forms fire blight cankers. Pathogen cells migrate inside the tree well ahead of visible symptoms. They can accumulate in susceptible tissue such as one-year old shoot tips and susceptible rootstocks causing infections distant from the original infection (Bogs et al. 1998). For example, in a Michigan study, E. amylovora moved an average of two inches per day in new growth and one-and-a-half inches per day in woody growth in five-year-old Gala, which equates to approximately 11 inches per week (Olive and Sundin 2023).
Erwinia amylovora can also infect susceptible one- and two-year-old tissue directly through wounds (e.g., insect feeding and hail injury) and natural openings (e.g., stomata, hydathodes) causing shoot blight infections (Vanneste 2000; Millet et al. 2022).

Cultural Controls
Plant on resistant rootstock
Resistant rootstocks (e.g., Geneva series for apples) can help prevent tree death from rootstock blight (Norelli et al. 2003; Russo et al. 2007). However, resistant rootstocks generally do not prevent the scion from becoming infected (Russo et al. 2007). Some data suggest that rootstock characteristics (root surface area and gene expression) can reduce the size of lesions which develop (Singh et al. 2019).
Winter sanitation
In winter, prune out old fire blight cankers as thoroughly as possible. Ideally, cut blight before you prune for tree structure so that the blighted cuttings can be removed from the orchard. Compared to cuts made in summer, winter removal cuts can be made closer to the visible canker edge. In winter the pathogen is confined to the cankered area. Cut at the next “horticulturally sensible” site below the canker to fulfill the requirements of the training system. You do not need to sterilize tools when you are cutting on fully dormant trees. Late dormant copper applications can enhance orchard sanitation, further reducing pathogen inoculum levels going into spring (Elkins et al. 2015).
Manage the orchard environment
In addition to warm temperatures, moisture is required to initiate infections. As little as two to three hours of wetting from dew or light rain is sufficient to trigger infection. Manage weeds and cover crops to limit relative humidity. Do not irrigate during bloom.
Flower removal in young blocks
Flower removal in young blocks and removal of late blooms limits the numbers of flowers and thus reduces potential points of infection. In two 2020 trials flower removal at pink bud stage for young nonbearing trees reduced infections to zero, compared to 71 to 77 per 100 clusters in water treated checks and 5 to 17 per 100 clusters in soluble copper treated trees (DuPont et al. 2022).
Keep tree vigor moderate
By limiting nitrogen fertilizer application, tree vigor is reduced. Moderating vigor will not prevent infection, but it can reduce damage to the tree by limiting the amount of new and more susceptible plant tissue.
Summer sanitation
Timely cutting of fire blight infected material soon after infections occur is recommended to reduce the spread of the pathogen throughout the orchard and to limit the advance of the disease in the tree, which can lead to plant death. Remove infected branches 12 to 18 inches below the visibly infected tissue in wood that is two years old or older (Figure 6). Timely removal reduces the number of trees that die from fire blight (DuPont et al. 2023). Moreover, this practice reduces the number of new infections which must be removed after initial pruning (DuPont et al. 2023). Although sanitizing pruning shears has been long considered important to prevent dissemination of fire blight infections (Van Der Zwet and Keil 1979), in multiple studies sterilizing shears made no difference in preventing canker formation as long as the cuts are made at the recommended distance below the visible canker (Travis and Kleiner 1997; Toussaint and Philion 2008; DuPont 2023). Canker removal cuts made four inches from where the branch joins structural wood (“ugly” stub cutting) can significantly reduce the number of cankers that redevelop on structural wood, which sustains fruiting area for future years (Steiner 2000; DuPont et al. 2023). While small cankers will reform on many of these cuts, these cankers can be removed during winter pruning. Breaking off infected flower clusters and diseased current season growth by hand can provide a rapid removal method, but it can result in a greater number of cankers in the orchard at the end of the season, with more cankers on structural wood (DuPont et al. 2023).
Applying a concentrated solution of Actigard as part of pruning therapies can reduce the severity of reoccurring fire blight cankers. In trees with Actigard applied, when removing fire blight infection, both the proportion of trees in which fire blight reoccurred and the rate of canker expansion was reduced in five years of field trials (Johnson and Temple 2016; Johnson and Temple 2017). Apply concentrated Actigard in water with an up and down motion to a one to one-and-a-half foot section of the central leader or major scaffold near where the fire blight infection was removed (Figure 7). Use the labeled rate of one ounce Actigard per one quart of water with one percent silicone-based penetrant.
Very young infected trees, first to third leaf, should be removed and destroyed. In young, vigorous trees, bacteria move quickly through the tree, and pruning therapies are unlikely to be effective. Apply Actigard to trees neighboring removed trees, to reduce canker occurrence risk.


Temperature Risk Models
The risk of fire blight infection during bloom can be calculated based on the temperature and moisture. In Washington Cougar Blight is available at WSU Decision Aid System for Tree Fruit (DAS) or in Excel format. This model calculates fire blight risk based on the temperature of the previous four days using the documented growth rate of the bacteria; for example, higher risk with multiple hours above 70°F (Pusey and Curry 2004). The model then projects risk for the next three days based on predicted temperatures. The model also includes an inoculum component that helps to estimate risk at specific sites. Maryblyt is another commonly used model for predicting fire blight risk (Turechek and Biggs 2015). Growers can use model information to decide when to spray. If trees are likely to be blooming during an upcoming high-risk period, protective sprays before an infection event are recommended (Smith and Pusey 2010).
Chemical Controls
Chemical Control Programs
There is a risk of fire blight infection any time there are open flowers on the tree, the weather is warm, and wetting occurs. Watch for and protect secondary flowers during the three weeks after petal fall, a common time for fire blight infection in Washington. Most sprays only protect the flowers that are open. Protect new flowers as they open. In warm weather follow-up sprays are needed every two to three days rotating the mode of action.
Conventional Management
Consider temperature and moisture to assess infection risk. After a period of warm weather (high infection risk) best results are obtained when antibiotics are applied within the 24-hour window before flower wetting (e.g., streptomycin, kasugamycin, oxytetracycline). Products used must contact the interior of the flowers in sufficient water and approved wetting agent to completely cover the tree crown interior. Antibiotic sprays every two to three days may be necessary during extended high or extreme risk periods. Rotate between materials with differing modes of action. In high risk blocks, or if fire blight was in the orchard last year, consider applications of Blossom Protect with Buffer Protect during early bloom. Lime sulfur applied for thinning is also antimicrobial and reduces blight pressure. See Tables 1 and 2.
Organic Management
Nonantibiotic control programs for fire blight which contain Blossom Protect and soluble copper products during the bloom period (e.g., Previsto, Cueva) followed by Bacillus based biorationals (e.g., Serenade Opti) at petal fall have performed well, suppressing fire blight with low russet risk in Oregon trials 2013 to 2021 (Johnson et al. 2022) as well as Washington and New York (Cox et al. 2015; Cox et al. 2016; DuPont et al. 2023). In difficult-to-thin apple varieties where multiple lime sulfur applications reduce Blossom Protect efficacy, soluble coppers can be an effective choice. Integrated programs with Blossom Protect or soluble coppers during high-risk bloom periods followed by essential oils or peracetic acid-peroxide products (e.g., Thyme Guard, Cinnerate, Oxidate 5.0, Jet-Ag) at petal fall have also had control similar to Blossom Protect/Serenade programs in several trials (DuPont et al. 2023). Consider drying times and rotations, and limit post petal fall applications of essential oils and peracetic acid-peroxides products to moderate russet risk. See Tables 1 and 2.
Non-bearing trees
Young nonbearing trees with high vigor in high-risk varieties or locations may require season-long protection from shoot blight. In recent trials for protection of young nonbearing trees, flower removal performed best followed by weekly applications of soluble copper at 3 to 4 quarts per acre or fixed copper at 1.5 pounds per acre (DuPont et al. 2022). Flower removal at pink for young nonbearing trees reduced infections to zero infections per 100 flower clusters in Pennsylvania and zero per 100 flower clusters in New York in 2020 trials (DuPont et al. 2022). Three applications of soluble copper (Previsto 3 qt., or Cueva 4 qt.) reduced infections per 100 flower clusters from 77 to 5.5 in New York and 71 to 17 in Pennsylvania, and three applications of fixed copper (1.5 lb) reduced infections per 100 flower clusters from 77 to 27 in New York and 71 to 8 in Pennsylvania (DuPont et al. 2022).
Apples – Conventional
Low to moderate risk
Cut and remove fire blight cankers. Good sanitation is essential for successful fire blight management.
-
Watch the model.• If an infection event is projected, apply an antibiotic within 24 hours before wetting.• Repeat every 2–3 days during warm, wet risk periods to cover newly opening flowers rotating FRAC.a• Continue weekly applications 1–2 weeks post petal fall.b
Apples – Conventional
High risk, high value varieties, history of blight
Cut and remove fire blight cankers. Good sanitation is essential for successful fire blight management.
- Use antibiotic mixes: oxytetracycline + kasugamycin or antibiotic + Actigard.a
- Cover every 2–3 days during warm conditions during bloom rotating FRAC.a
- Acidify spray tanks to 5.5 to improve antibiotic efficacy. New research shows 4.0 may further improve efficacy.c
- Continue weekly applications 1–2 weeks post petal fall.b
Apples – Organic
Cut and remove fire blight cankers. Good sanitation is essential for successful fire blight management.
-
Blossom Protect + Buffer Protect early.d
-
Lime sulfur (+ oil).
-
Blossom Protect + Buffer Protect.
-
Depending on the model and cultivar russet risk/drying conditions, soluble copper (Previsto 3 qt, Cueva 4 qt, Cueva 3 qt + Serenade Opti, Instill, Mastercop).
-
Petal fall + 1–2 weeks Serenade Opti/Aso (most fruit safe) or 2% lime sulfur (red apples), essential oils (Cinnerate, Thyme Guard), peracetic acids (Oxidate 5.0, Jet-Ag).e,f,g
Apples – Organic
Hard to thin varieties, short bloom period
Cut and remove fire blight cankers. Good sanitation is essential for successful fire blight management.
- Lime sulfur (+ oil) 2–3 applications.
- Depending on the model and cultivar russet risk/drying conditions, soluble copper (Previsto 3 qt, Cueva 4 qt, Cueva 3 qt + Serenade Opti, Instill, Mastercop).g
- Petal fall + 1–2 weeks Serenade Opti/Aso (most fruit safe) or 2% lime sulfur (red apples), essential oils (Cinnerate, Thyme Guard), peracetic acids (Oxidate 5.0, Jet-Ag).e,g
Apples – Organic
Hard to thin varieties, long bloom period
Cut and remove fire blight cankers. Good sanitation is essential for successful fire blight management.
- Lime sulfur (+ oil).
- Blossom Protect + Buffer Protect.
- Lime sulfur + oil.
- Blossom Protect + Buffer Protect.
- Depending on the model and cultivar russet risk/drying conditions, soluble copper (Previsto 3 qt, Cueva 4 qt, Cueva 3 qt + Serenade Opti, Instill, Mastercop).
- Petal fall + 1–2 weeks Serenade Opti/Aso (most fruit safe) or 2% lime sulfur (red apples), essential oils (Cinnerate, Thyme Guard), peracetic acids (Oxidate 5.0, Jet-Ag).e
a Rotate modes of action using fungicide resistance action committee (FRAC) codes. Rotation is necessary to prevent emergence of resistant E. amylovora. Rotate as necessary to comply with application intervals for individual products. Do not apply Actigard at closer than seven-day interval (label restriction).
b Kasumin 2L. Do not make more than four applications per year. Post petal fall restriction has been removed (March 2021).
c Spray tank acidification has increased efficacy of oxytetracycline products (e.g., Mycoshield).
d Blossom Protect yeast need about 12 hours to grow on the flower to protect against infection before a wetting event.
e Lime sulfur at this timing can interfere with oil sprays for mites.
f Blossom Protect+ Buffer Protect, then Previsto (full bloom), then Serenade Opti/Aso (petal fall) was the best organic combination in 13 trials in Oregon at 83% relative control (Johnson et al. 2022) similar to antibiotics.
g Consider drying conditions. Fruit marking may occur under slow drying conditions.
Pears – Conventional
Low to moderate risk
Cut and remove fire blight cankers. Good sanitation is essential for successful fire blight management.
- Watch the model.
- If an infection event is projected apply an antibiotic less than 24 hours before wetting.
- Repeat every 2–3 days during warm, wet risk periods to cover newly opening flowers rotating FRAC.a
- Continue weekly applications 1–2 weeks post petal fall during warm wet risk periods.b
Pears – Conventional
High risk, sensitive varieties, history of blight
Cut and remove fire blight cankers. Good sanitation is essential for successful fire blight management.
- Use antibiotic mixes: oxytetracycline + kasumamycin or antibiotic + Actigard.a
- Cover every 2 days during warm conditions during bloom, rotating FRAC.a
- Acidify spray tanks to at least 5.5 to improve antibiotic efficacy. New research shows that 4.0 may further improve efficacy.c
- Continue weekly applications 1–2 weeks post petal fall.b
Pears – Organic
Easy to mark varieties (Anjou/ Comice)
Cut and remove fire blight cankers. Good sanitation is essential for successful fire blight management.
- 2 applications of Blossom Protect + Buffer Protect during early bloom to petal fall (70%–80% bloom if single treatment).d
- Follow with Serenade Opti/Aso at petal fall to reduce russet risk from Blossom Protect yeast.
Pears – Organic
Marking tolerant varieties (Bosc)
Cut and remove fire blight cankers. Good sanitation is essential for successful fire blight management.
- • 2 applications of Blossom Protect + Buffer Protect during early bloom to petal fall (70%–80% bloom if single treatment).
- Follow with soluble copper (Cueva 4 qt, Previsto 3 qt) if the model indicates risk (warm/wet).
- Petal fall plus options also include essential oils (Cinnerate, Thyme Guard), peracetic acids (Oxidate 5.0, Jet-Ag).
a Rotate modes of action. Rotation is necessary to prevent emergence of antibiotic resistant E. amylovora. Rotate as necessary to comply with application intervals for individual products. Do not apply Actigard at closer than seven-day interval (label restriction).
b Kasumin 2L. Do not make more than four applications per year. The post petal fall restriction has been removed (March 2021).
c Spray tank acidification has increased efficacy of oxytetracycline products (e.g., Mycoshield).
d Blossom Protect yeast need about 12 hours to grow on the flower to protect against infection before a wetting event.
Antibiotic Resistance Management
Fire blight pathogen resistance to streptomycin was first detected in California in 1971 and in Oregon and Washington in the 1970s and 1980s (Coyier and Covey 1975). It was believed to be widespread in the western United States in 1991 and in other parts of the US including Idaho, Utah, Missouri, Michigan (2003), and New York (2002, 2016, 2021) (Loper et al. 1991a; McGhee and Sundin 2011; Forster et al. 2015a; Tancos et al. 2016). In an ongoing antibiotic resistance survey, researchers did not find isolates from collected samples to be resistant to streptomycin and tetracycline, but some isolates exhibited resistance and tolerance to kasugamycin (Zhao 2023). To minimize the risk of resistance development, it is recommended to apply streptomycin in combination with oxytetracycline and limit streptomycin application to once per season. Kasugamycin could be applied in a mixture with oxytetracycline or rotation with oxytetracycline.
Strategies for Improving Protective Programs
Coverage
Product efficacy is based on thorough coverage of flowers. Use tree row volume to apply appropriate volumes to cover the tree architecture in your orchard. Products applied every other row or at high speeds may have insufficient coverage and lower efficacy.
Timing
Antibiotics have the highest efficacy when applied shortly before a moisture event. Nonetheless, kasugamycin and streptomycin can also be applied up to 12 hours after a moisture event, but with reduced effectiveness. Streptomycin has locally systemic activity and kasugamycin is effective on bacteria which have been washed into the floral cup but have not yet invaded the flower.
pH of Spray Tank Water
It is important to appropriately acidify spray tank water when using antibiotics (especially oxytetracycline and kasugamycin). Antibiotic efficacy reported in WSU trials is with spray tank water buffered to pH 5.6. At higher pH the antibiotic degradation rate is faster and thus efficacy is often lower. For example, in one trial kasugamycin reduced the bacterial population by 86% to 96% at pH 5.1 but only 21% to 35% at pH 7.3 (Adaskaveg et al. 2011). Johnson and KC (2021) found that buffering oxytetracycline products to a pH 4 can further improve efficacy.
Use Appropriate Rates
Quantity of active ingredient is important to obtain efficacy. For example, recent work looking at rates of copper products demonstrated that as metallic copper content increases, copper product efficacy increases up to approximately 0.2 lb metallic copper per 100 gal per acre (DuPont 2019) (Figure 8). This is equivalent to approximately 3 qt of Previsto or 4 qt of Cueva per acre.
Mixtures
A full rate of kasugamycin (100 ppm) with a full rate of oxytetracycline (200 ppm), as well as streptomycin (100 ppm) mixed with a full rate of oxytetracycline (200 ppm) have provided improved efficacy in some trials (Oregon 2015–2018) (Johnson, unpublished data). Actigard (2 oz per 100 gal) plus an antibiotic applied during bloom has improved the efficacy of antibiotics an average of 5% to 6% per application of Actigard in trials in Washington and Oregon (Johnson et al. 2016).
Antibiotics
Kasugamycin (Kasumin) is a recently labeled antibiotic that provides excellent levels of control. For example, in eight trials in Michigan relative control averaged 92% (Sundin and McGhee 2010). In an ongoing antibiotic resistance survey in Washington, some isolates exhibited resistance and tolerance to kasugamycin (Zhao 2023) but no field level failures were reported. There is an intermediate risk of resistance developing to this antibiotic (Adaskaveg et al. 2011). Kasumin controls streptomycin-resistant strains of fire blight. Kasumin provides forward control for two to three days prior to rain events (on flowers open when applied) and will be partially effective for blossom blight control if applied within 12 hours after a rain event. Kasumin is not locally systemic like streptomycin. Thus, Kasumin will not penetrate into the nectaries and will not be able to control an infection once the fire blight pathogen reaches the nectaries. Acidifying spray tanks (target 5) is important to reduce antibiotic break down and extend activity. Kasugamycin is ultraviolet (UV) sensitive, and spraying at night and reduced pH can help improve residual time. The use of a nonionic surfactant enhances deposition of the antibiotic on flowers.
Oxytetracycline (Mycoshield, FireLine) generally provide good levels of control in Washington trials (average 74% control) and has a low risk of resistance development (DuPont et al. 2023). Oxytetracycline products should be applied within 12 to 24 hours prior to a moisture event for best results. Oxytetracycline is considered bacteriostatic (inhibits bacterial growth). Thus, to be effective it must be applied prior to wetting events where it can prevent growth on stigmas. Oxytetracycline is also sensitive to UV degradation, and much of the activity is lost within one to two days after application. Acidifying spray tanks (target pH 5) is important to reduce antibiotic break down and extend activity. The use of a nonionic surfactant enhances deposition of the antibiotic on flowers.
Streptomycin (Agri-Mycin, FireWall) resistant strains of the fire blight pathogen have been present in Washington orchards since 1975 (Coyier and Covey 1975; Loper et al. 1991b). Recent tests have indicated that the proportion of the pathogen population resistant to this antibiotic has dropped and expected control levels have improved (Forster et al. 2015). In an ongoing survey in Washington no fire blight strains resistant to streptomycin were detected (Zhao 2023). This product should only be used in combination with oxytetracycline and should not be used unless a high-to-extreme risk infection period is expected. Limit use to once per season. Repeat application has a high risk of resistant bacteria being uncontrolled. The use of a nonionic surfactant enhances deposition of the antibiotic in and on flowers. Acidifying spray tanks (target pH 5) is important to reduce antibiotic break down and extend activity.
Non-Antibiotic Products
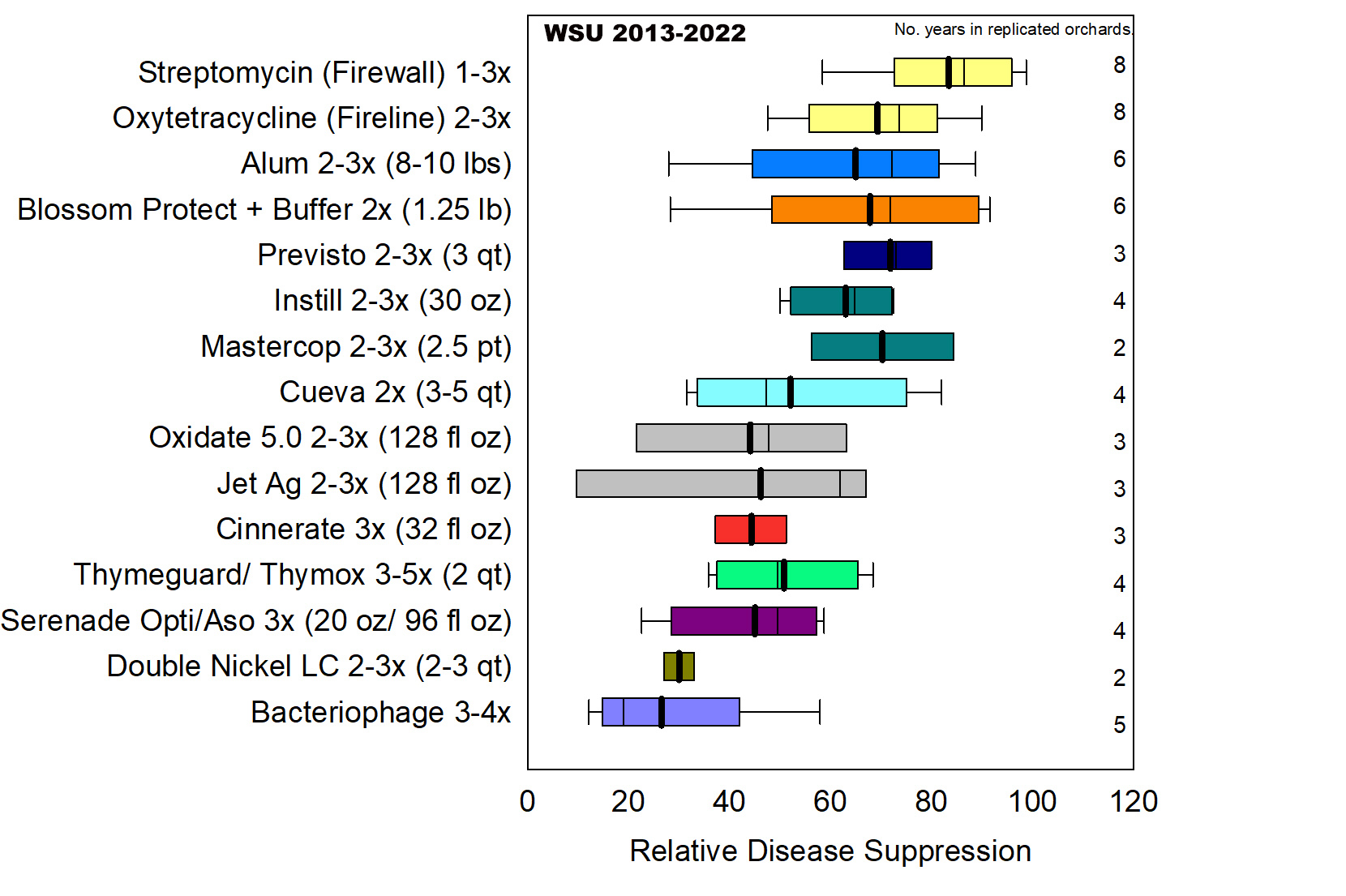
In summary analysis of eight Washington trials Alum (potassium aluminum sulfate), Blossom Protect (A. pullulans) and several copper products (Previsto, Mastercop, Instill) provided good disease suppression of 70% to 73% similar to antibiotic treatments (DuPont et al. 2023) (Figure 9). Several essential oil, copper, peracetic acid-peroxide and biological products (Serenade Opti, Cueva, Oxidate 5.0, Jet-Ag, Thyme Guard, Thymox and Cinnerate) provided intermediate disease suppression between 45% to 62% significantly better than the water-treated trees.
*Important! Some of the pesticides discussed in this presentation were tested under an experimental use permit granted by WSDA. Application of a pesticide to a crop or site that is not on the label is a violation of pesticide law and may subject the applicator to civil penalties up to $7,500. In addition, such an application may also result in illegal residues that could subject the crop to seizure or embargo action by WSDA and/or the U.S. Food and Drug Administration. It is your responsibility to check the label before using the product to ensure lawful use and obtain all necessary permits in advance.
Blossom Protect
Blossom Protect is a combination of two strains of Aureobasidium pullulans, a yeast that occurs naturally in the Pacific Northwest pome fruit flowers. It is applied in combination with a buffer, Buffer Protect. Relative efficacy of Blossom Protect plus Buffer Protect in Washington averaged 72% across six orchard trials with two to three applications (DuPont et al. 2023). High relative efficacy has also been documented in Oregon with control comparable to antibiotic standards, in Michigan where two applications resulted in between 85% and 92% relative control, and in Germany where Blossom Protect provided an average of 79% efficacy across 11 trials (Kunz et al. 2011; Johnson and Temple 2013; Sundin et al. 2018; Outwater et al. 2019).
Recommendations for Blossom Protect use include applications to every tree row (Temple et al. 2020). It may also be important to apply with at least 12 to 24 hours between application and an impending rain event in order to give sufficient time for A. pullulans populations to colonize flowers (Temple et al. 2020).
Coppers
Coppers are generally effective disease control products. Free copper ions are taken up by pathogen cells and cause toxicity by nonselectively denaturing proteins in cells. New soluble copper formulations are designed to have lower plant phytotoxicity. For example, copper octanoate (Cueva) is a copper soap, copper hydroxide product (Previsto) is formulated with a polymer matrix designed to lessen toxicity of copper ions on the plant surface, and copper sulfate pentahydrate products (e.g., Instill) are generally highly soluble but may be formulated to reduce plant phytotoxicity. Soluble coppers are used during bloom in semiarid Washington but can cause phytotoxicity in wetter areas in Oregon and California (Smith 2012, 2015; Johnson 2016).
In Washington trials relative disease suppression resulting from use of soluble coppers (e.g., Previsto, Instill, Mastercop, Cueva) ranged from a median of 47% to 73% in 2013 to 2022 trials (DuPont et al. 2023). These results are similar to field trials from other regions of the United States, such as New York, Virginia, and Oregon, where soluble coppers provided between 50% and 80% relative control, depending on type, timing, and rate of application (Yoder et al. 2013; Cox et al. 2014; Choi et al. 2018; DeShields and DeShields 2020). The amount of metallic copper in a product will determine in part the appropriate rate for optimum efficacy. Analysis based on metallic copper content of copper products combined over multiple years and products indicates an optimum range of metallic copper application for fire blight control between 0.16 and 0.25 lb per 100 gal per acre of metallic copper equivalent (p < 0.001; R2 = 0.46) (DuPont 2019) (Figure 8).
Concern about fire blight resistance to coppers has been raised due in part to resistance documented in Pseudomonas syringae strains affecting almonds (Andersen et al. 1991). However, none of 138 isolates tested in Washington in 1991 by Loper et al. (1991a) grew in copper at 0.16 mM, while 15 of 80 isolates in nearby British Columbia, Canada, had a reduced response at 0.16 mM. No tolerance was found at field rates of 1.10 mM (Sholberg et al. 2001).
Due to relatively high disease suppression rates, soluble coppers fit well in an integrated nonantibiotic program following early bloom Blossom Protect plus Buffer Protect applications (Johnson et al. 2022).
Bacillus Products
Serenade Opti/Aso is considered a “fruit safe” material, made by fermenting a strain of Bacillus subtilis. The antimicrobial activity of Serenade comes primarily from biochemical compounds produced by the bacterium during fermentation. Bacillus subtilis strain QST 713 (Serenade) has provided an average of 60% control in Oregon and is best used as part of an integrated program (Smith 2012; Johnson and Temple 2013; Smith 2015). In four Washington trials from 2014 to 2022 Serenade Opti/Aso provided 50% median relative control (DuPont et al. 2023).
Oxidizing Agents
Several peracetic acid-peroxide products are available (e.g., Jet-Ag, Oxidate 5.0) for fire blight. These low residue oxidative agents have the potential to damage microbial structures, such as membrane layers, and disrupt microbial cellular processes, like DNA or protein synthesis, leading to cell death (Wagner et al. 2002; Finnegan et al. 2010). Oxidizing agents (Jet-Ag and Oxidate 5.0) produced median relative disease suppression of 53% and 62% in Washington with two to three applications post inoculation (four trials: 2019–2022). When multiple post petal fall applications were made under slow-drying conditions, fruit marking occurred. In order to limit fruit marking potential, peracetic acid-peroxide products should be applied only in fast-drying conditions and up until the early post petal fall period.
Plant Extracts
Plant essential oils of thyme and cinnamon have antibacterial activity against E. amylovora (Chizzola et al. 2008; Kokoskova et al. 2011; Tawfik and Shahin 2011; Akhlaghi et al. 2020). In Washington trials essential oil products Cinnerate, Thyme Guard and Thymox provided a median of 45% to 49% relative disease suppression comparable to recent trials in other states (DeShields and DeShields 2020; DuPont et al. 2023).
Other Biologicals
Bacteriophages are viruses that kill bacteria (Meile et al. 2017). Bacteriophages are an attractive option for integrated disease management due to their target specificity; however, sensitivity to environmental conditions have thus far made application challenging (Gill and Abedon 2003; Nagy et al. 2012; Nagy et al. 2015). In Washington trials, relative disease suppression of bacteriophage products against E. amylovora has been variable, with control below 20% observed in 2019 and 2020, while in 2022 three applications provided 58% relative disease suppression (DuPont et al. 2023). This variability has also been seen in other regions. For example bacteriophages provided 35% and 39% relative suppression in Michigan 2019 trials and 74% and 42% in 2018 trials (Sundin et al. 2018; Outwater et al. 2019).
Plant Defense Inducers
Acibenzolar-S-methyl (Actigard 50WG) is a synthetic inducer of systemic acquired resistance (SAR). Its mode of action mimics the plant hormone salicylic acid, which is responsible for priming the plant’s defense system. The level of protection is smaller compared to an antibiotic, but it lasts longer—approximately a week (Maxson-Stein et al. 2002). Actigard (two oz per acre) plus an antibiotic applied during bloom improved the efficacy of antibiotics an average of 5% to 6% per application in trials in Washington and Oregon (Johnson et al. 2016). Therapeutic applications of Actigard to a one-foot section of the scaffold where fire blight cankers were removed have reduced the size of canker expansion and the number of trees which died (Johnson and Temple 2016; Johnson and Temple 2017).
Apple Materials
Excerpt from the WSU Crop Protection Guide. For timings at which each pesticide can be used refer to the Crop Protection Guide.
d = dormant; dd= delayed dormant; pp= prepink/pink; b = bloom; pb = post bloom; sf = shuck fall; es = early summer (14-32 after full bloom); s = summer; ls = late summer; ph = preharvest; h = harvest; ah = after harvest
Pear Materials
Excerpt from the WSU Crop Protection Guide. For timings at which each pesticide can be used refer to the Crop Protection Guide.
d = dormant; dd= delayed dormant; pp= prepink/pink; b = bloom; pb = post bloom; sf = shuck fall; es = early summer (14-32 after full bloom); s = summer; ls = late summer; ph = preharvest; h = harvest; ah = after harvest
Additional Resources
Visit for the recent model projections of blossom blight risk at your site.
Crop Protection Guide recommendations are updated on an annual basis.
Cutting Fire Blight Infections
News article May 2023.
Organic Fire Blight Management in Western US
eOrganic article.
Plan for Multiple Fire Blight Conditions, Be Agile
Example programs for high and low risk blocks. WSU Newsletter article, Updated March 2022.
Fire blight cankers left in the orchard are the source for new spring infections. WSU Newsletter article, February 2022.
Fire Blight Susceptibility of Apple Cultivars
Written by: Sarah Kostick, WSU Horticultural Doctoral Candidate. May 2019.
Tips for Using Blossom Protect
WSU Newsletter article April 10, 2017.
Product Efficacy Trials
Washington Fire Blight Product Efficacy 2016-2024 Report (u)
DuPont Cox Peter Johnson Integrated Fire Blight Management Final Report (f)
WSU Fire Blight New Product Efficacy Trials 2020
Fire Blight Management New Products and Effective Rates Fresh Pear Final Report 2019
WSU Fire Blight New Product Efficacy 2019
Integrated Fire Blight Management WSTFRC YR2 Report
Integrated Fire Blight Management WSTFRC YR1 Report
Literature Cited
Adaskaveg, J.E., H. Forster, and M.L. Wade. 2011. Effectiveness of Kasugamycin Against Erwinia amylovora and Its Potential Use for Managing Fire Blight of Pear. Plant Disease 95: 448–454.
Akhlaghi, M., S. Tarighi, and P. Taheri. 2020. Effects of Plant Essential Oils on Growth and Virulence Factors of Erwinia amylovora. Journal of Plant Pathology 11.
Andersen, G.L., O. Menkissoglou, and S.E. Lindow. 1991. Occurrence and Properties of Copper-Tolerant Strains of Pseudomonas-syringae Isolated from Fruit Trees in California. Phytopathology 81: 648–656.
Biggs, A.R. 1994. Characteristics of Fire Blight Cankers Following Shoot Inoculations of Three Apple Cultivars. Hortscience 29: 795–797.
Bluhm, B., and J. Stover. 2016. Fire Blight: An Emerging Problem for Blackberry Growers in the Mid South. North American Bramble Growers Research Foundation 2016 Report.
Bogs, J., I. Bruchmuller, C. Erbar, and K. Geider. 1998. Colonization of Host Plants by the Fire Blight Pathogen Erwinia amylovora Marked with Genes for Bioluminescence and Fluorescence. Phytopathology 88: 416–421.
Chizzola, R., H. Michitsch, and C. Franz. 2008. Antioxidative Properties of Thymus vulgaris Leaves: Comparison of Different Extracts and Essential Oil Chemotypes. Journal of Agricultural and Food Chemistry 56: 6897–6904.
Choi, M., K.M. Ayer, and K.D. Cox. 2018. Evaluation of Bactericide Programs for the Management of Fire Blight on ‘Gala’ Apples in NY, 2018. Plant Disease Management Reports 13.
Cox, K.D., S.M. Villani, and K. Ayer. 2016. Evaluation of Bactericide and Chemical Regulator Programs for the Management of Fire Blight on ‘Idared’ Apples in NY, 2015. Plant Disease Management Reports. PF014.
Cox, K.D., S.M. Villani, and K.A. Bekoscke. 2014. Evaluation of Bactericide and Chemical Regulator Programs for Management of Fire Blight on ‘Idared’ Apples in NY, 2014. Plant Disease Management Reports 9.
Cox, K.D., S.M. Villani, and K. Bekoscke. 2015. Evaluation of Bactericide and Chemical Regulator Programs for the Management of Fire Blight on ‘Idared’ Apples in NY, 2014. Plant Disease Management Report. PF023.
Coyier, D.L., and R.P. Covey. 1975. Tolerance of Erwinia amylovora to Streptomycin Sulfate in Oregon and Washington. Plant Disease 59:849–852.
DeShields, J.B., and A.N.K. DeShields. 2020. Evaluation of Organic Materials for Fire Blight Management in Pears, 2020. Plant Disease Management Reports 15.
DuPont, S.T. 2019. Fire Blight Management: New Products and Effective Rates. In Final Project Report. Washington State Tree Fruit Research Commission.
DuPont, S.T., K. Cox, K. Johnson, K. Peter, T. Smith, M. Munir, and A. Baro. 2023. Evaluation of Biopesticides for the Control of Erwinia amylovora in Apple and Pear. Journal of Plant Pathology 106: 889–901.
DuPont, S.T., K. Cox, K. Peter, and K. Johnson. 2022. Integrated Fire Blight Management. In Final Project Report. Washington State Tree Fruit Research Commission.
DuPont, S.T., M. Munir, K. Cox, K. Johnson, K. Peter, and A. Baro. 2023. Evaluation of Pruning Therapies in Apple Trees with Fire Blight. Journal of Plant Pathology 105: 1695–1709.
Elkins, R.B., T.N. Temple, C.A. Shaffer, C.A. Ingels, S.B. Lindow, B.G. Zoller, and K.B. Johnson. 2015. Evaluation of Dormant-Stage Inoculum Sanitation as a Component of a Fire Blight Management Program for Fresh-Market Bartlett Pear. Plant Disease 99: 1147–1152.
Finnegan, M., E. Linley, S.P. Denyer, G. McDonnell, C. Simons, and J. Maillard. 2010. Mode of Action of Hydrogen Peroxide and Other Oxidizing Agents: Differences Between Liquid and Gas Forms. Journal of Antimicrobial Chemotherapy 65: 2108–2115.
Forster, H., G.C. McGhee, G.W. Sundin, and J.E. Adaskaveg. 2015. Characterization of Streptomycin Resistance in Isolates of Erwinia amylovora in California. Phytopathology 105: 1302–1310.
Gill, J., and S.T. Abedon. 2003. Bacteriophage Ecology and Plants. APS Net Feature. DOI: 10.1094/APSnetFeature-2003-1103.
Johnson, K. n.d. Oregon State University.
Johnson, K.B. 2000. Fire Blight of Apple and Pear. Plant Health Instructor.
Johnson, K. 2016. Non-Antibiotic Fire Blight Control That Minimizes Fruit Russet Risk. Tree Fruit Research Commission.
Johnson, K.B., and A. KC. 2021. Refinement of Practical Fire Blight Control: Buffered Oxytetracycline. Washington State Tree Fruit Research Commission Final Report.
Johnson, K.B., T.J. Smith, T.N. Temple, E. Gutierrez, R.B. Elkins, and S.P. Castagnoli. 2016. Integration of Acibenzolar-S-Methyl with Antibiotics for Protection of Pear and Apple from Fire Blight caused by Erwinia amylovora. Crop Protection 88: 149–154.
Johnson, K.B., V.O. Stockwell, D.M. Burgett, D. Sugar, and J.E. Loper. 1993. Dispersal of Erwinia amylovora and Pseudomonas fluorescens by Honey Bees from Hives to Apple and Pear Blossoms. Phytopathology 83: 478–484.
Johnson, K.B., and T.N. Temple. 2013. Evaluation of Strategies for Fire Blight Control in Organic Pome Fruit Without Antibiotics. Plant Disease 97: 402–409.
Johnson, K.B., and T.N. Temple. 2016. Comparison of Methods of Acibenzolar-S-Methyl Application for Post-Infection Fire Blight Suppression in Pear and Apple. Plant Disease 100: 1125–1131.
Johnson, K.B., and T.N. Temple. 2017. Induction of Systemic Acquired Resistance Aids Restoration of Tree Health in Field-Grown Pear and Apple Diseased with Fire Blight. Plant Disease 101: 1263–1268.
Johnson, K.B., T.N. Temple, A. Kc, and R.B. Elkins. 2022. Refinement of Nonantibiotic Spray Programs for Fire Blight Control in Organic Pome Fruit. Plant Disease 106: 623–633.
Kokoskova, B., D. Pouvova, and R. Pavela. 2011. Effectiveness of Plant Essential Oils Against Erwinia amylovora, Pseudomonas syringae pf Syringae and Associated Saprophytic Bacteria on/in Host Plants. Journal of Plant Pathology 93: 133.
Kunz, S., A. Schmitt, and P. Haug. 2011. Development of Strategies for Fire Blight Control in Organic Fruit Growing. Acta Hort 896: 431–436.
Loper, J., M. Henkels, R. Roberts, G. Grove, and M. Willet. 1991a. Evaluation of Streptomycin, Oxytetracycline, and Copper Resistance of Erwinia amylovora Isolated from Pear Orchards in Washington State. Plant Disease 75: 287–290.
Loper, J.E., M.D. Henkels, R.G. Roberts, G.G. Grove, M.J. Willett, and T.J. Smith. 1991b. Evaluation of Streptomycin, Oxytetracycline, and Copper Resistance in Erwinia amylovora Isolated from Pear Orchards in Washington State. Plant Disease 75: 287–290.
Maxson-Stein, K., S.Y. He, R. Hammerschmidt, and A.L. Jones. 2002. Effect of Treating Apple Trees with Acibenzolar-S-Methyl on Fire Blight and Expression of Pathogenesis-Related Protein Genes. Plant Disease 86: 785–790.
McGhee, G.C., and G.W. Sundin. 2011. Evaluation of Kasugamycin for Fire Blight Management, Effect on Nontarget Bacteria, and Assessment of Kasugamycin Resistance Potential in Erwinia amylovora. Phytopathology 101: 192–204.
Meile, S., J. Du, M. Dunne, S. Kilcher, and M. Loesser. 2017. Engineering Therapeutic Phages for Enhanced Antibacterial Efficacy. Current Opinion in Virology 52: 182–191.
Millet, F., Z. Cui, K. Miller, Q. Zeng. 2022. Glandular and Non-Glandular Trichomes Are Colonization Sites and Host Entry Points of the Fire Blight Pathogen on Apple Leaves. In International Symposium on Fire Blight of Rosaceous Plants, Dresden-Pillnitz Germany.
Momol, M.T., and S. Aldwincklke. 2000. Genetic Diversity and Host Range of Erwinia amylovora. In Fire Blight: The Disease and Its Causative Agent, Erwinia amylovora, J.L. Vanneste, ed. CABI, New Zealand.
Momol, M.T., J.L. Norelli, D.E. Piccioni, E.A. Momol, H.L. Gustafson, J.N. Cummins, and H.S. Aldwinckle. 1998. Internal Movement of Erwinia amylovora Through Symptomless Apple Scion Tissues into the Rootstock. Plant Disease 82: 646–650.
Nagy, J.K., L. Kiraly, and S. Ildiko. 2012. Phage Therapy for Plant Disease Control with a Focus on Fire Blight. Central European Journal of Biology 7: 1–12.
Nagy, J.K., I. Schwarczinger, A. Kunstler, M. Pogany, and L. Kiraly. 2015. Penetration and Translocation of Erwinia amylovora-Specific Bacteriophages in Apple—A Possibility of Enhanced Control of Fire Blight. European Journal of Plant Pathology 142: 815–827.
Norelli, J.L., H.T. Holleran, W.C. Johnson, T.L. Robinson, and H.S. Aldwinckle. 2003. Resistance of Geneva and Other Apple Rootstocks to Erwinia amylovora. Plant Disease 87: 26–32.
Ogawa, J., and H. English. 1991. Diseases of Temperate Zone Tree Fruit and Nut Crops. University of California Division of Agriculture and Natural Resources Publication 3345.
Olive, K., and G.W. Sundin. 2023. Quantitative Analysis of Erwinia amylovora Population Dynamics During Apple Shoot Infection. Phytopathology 113.
Outwater, C.A., T.J. Proffer, S.M. Slack, and G.W. Sundin. 2019. Evaluation of New and Existing Biological Control Materials for the Control of Fire Blight on Gala Apples, 2019. Plant Disease Management Reports 14: 1.
Pattemore, D.E., R.M. Goodwin, H.M. McBrydie, S.M. Hoyte, and J.L. Vanneste. 2014. Evidence of the Role of Honey Bees (Apis mellifera) as Vectors of the Bacterial Plant Pathogen Pseudomonas syringae. Australian Plant Pathology 43: 571–575.
Pusey, P.L., and E.A. Curry. 2004. Temperature and Pomaceous Flower Age Related to Colonization by Erwinia amylovora and Antagonists. Phytopathology 94(8): 901–911.
Russo, N.L., T.L. Robinson, G. Fazio, and H.S. Aldwinckle. 2007. Field Evaluation of 64 Apple Rootstocks for Orchard Performance and Fire Blight Resistance. Hortscience 42: 1517–1525.
Santander, R.D., K. Gašić, and S.G. Aćimović. 2022a. Selective Quantification of Erwinia amylovora Live Cells in Pome Fruit Tree Cankers by Viability Digital PCR. In Plant Pathology: Method and Protocols, N. Luchi, ed., 231–249. Springer.
Santander, R.D., F. Khodadadi, C.L. Meredith, A. Radenovic, J. Clements, and S.G. Acimovic. 2022b. Fire Blight Resistance, Irrigation and Conducive Wet Weather Improve Erwinia amylovora Winter Survival in Cankers. Frontiers Microbiology 13.
Sholberg, P.L., K.E. Bedford, P. Haag, and P. Randall. 2001. Survey of Erwinia amylovora Isolates from British Columbia for Resistance to Bactericides and Virulence on Apple. Canadian Journal of Plant Pathology—Revue Canadienne De Phytopathologie 23: 60–67.
Singh, J., J. Fabrizio, E. Desnoues, J.P. Silva, W. Busch, and A. Khan. 2019. Root System Traits Impact Early Fire Blight Susceptibility in Apple (Malus × domestica). BMC plant biology 19: 579.
Slack, S.M., Q. Zeng, C.A. Outwater, G.W. Sundin, S.M. Slack, C.A. Outwater, and G.W. Sundin. 2017. Microbiological Examination of Erwinia amylovora Exopolysaccharide Ooze. Phytopathology 107(4): 403–411.
Smith, T. 2012. Improving the Management of Two Critical Pome Fruit Diseases. Tree Fruit Research Commission Final Report.
Smith, T. 2015. Improving the Management of Two Critical Pome Fruit Diseases. Tree Fruit Research Commission Final Report.
Smith, T.J., and P.L. Pusey. 2010. CougarBlight 2010, a Significant Update of the CougarBlight Fire Blight Infection Risk Model. ISHS Acta Horticulturae 896: XII International Workshop on Fire Blight: 331–338.
Steiner, P.W. 2000. Integrated Orchard and Nursery Management for the Control of Fire Blight. In Fire Blight: The Disease and Its Causative Agent Erwinia amylovora, J.L. Vannesta, ed., 339–358. CABI Publishing.
Sundin, G.W., and G.C. McGhee. 2010. Kasumin: Field Results for Fire Blight Management and Evaluation of the Potential for Resistance Development in Erwinia amylovora. Phytopathology 100: S166–S166.
Sundin, G.W., C.A. Outwater, S.M. Slack, and M.R. Dobbins. 2018. Evaluation of Biological Control Materials for the Control of Fire Blight on Buckeye Gala Apples in Michigan, 2018. Plant Disease Management Reports 13: 1.
Tancos, K.A., S. Villani, S. Kuehne, E. Borejsza-Wysocka, D. Breth, J. Carol, H.S. Aldwinckle, and K.D. Cox. 2016. Prevalence of Streptomycin-Resistant Erwinia amylovora in New York Apple Orchards. Plant Disease 100: 802–809.
Tawfik, A.E., and H.A. Shahin. 2011. Efficacy of Some Plant Extracts on the Growth of Streptomycin Resistant and Sensitive Isolates of Erwinia amylovora. Acta Horticulturae 896: 477–482.
Temple, T., E. Thompson, S. Uppala, D. Granatstein, and K. Johnson. 2020. Floral Colonization Dynamics and Specificity of Aureobasidium pullulans Strains Used to Suppress Fire Blight of Pome Fruit. Plant Disease 104: 121–128.
Teviotdale, B.L. 2011. Fire Blight. UC Statewide Integrated Pest Management Program. University of California.
Thomson, S.V. 1986. The Role of the Stigma in Fire Blight Infections. Phytopathology 76: 476–482.
Toussaint, V., and V. Philion. 2008. Natural Epidemic of Fire Blight in a Newly Planted Orchard and Effect of Pruning on Disease Development. Acta Horticulturae 793: 313–320.
Travis, J.W., and W.C. Kleiner. 1997. Evaluation of Techniques for Removal of Fire Blighted Shoots. Pennsylvania Fruit News 77.
Tullis, E.C. 1929. Studies on the Overwintering and Modes of Infection of the Fire Blight Organism. Michigan State College of Agriculture and Applied Science.
Turechek, W.W., and A.R. Biggs. 2015. Maryblyt v. 7.1 for Windows: An Improved Fire Blight Forecasting Program for Apples and Pears. Plant Health Progress 16–22.
van der Zwet, T., and S.W. Beer. 1991. Fire Blight—Its Nature, Prevention and Control: A Practical Guide to Integrated Disease Management. US Department of Agriculture.
Van Der Zwet, T., and H.L. Keil. 1979. Fire Blight: A Bacterial Disease of Rosaceous Plants. US Department of Agriculture.
Vanneste, J.L. 2000. What Is Fire Blight? Who Is Erwinia amylovora? How To Control It? In Fire Blight: The Disease and Its Causative Agent, Erwinia amylovora, J.L. Vannesta, ed., 1–6. CABI Publishing.
Wagner, M., D. Brumelis, and R. Gehr. 2002. Disinfection of Wastewater by Hydrogen Peroxide or Peracetic Acid: Development of Procedures for Measurement of Residual Disinfectant and Application to a Physicochemically Treated Municipal Effluent. Water Environment Research 74.
Yoder, K.S., A.E. Cochran, W.S. Royston, and S.W. Kilmer. 2013. Blossom Blight, Scab Control and Russet Effects by Coppers on Gala Apple, 2013. Plant Disease Management Reports 8.
Zhao, Y. 2023. Comprehensive Monitoring and Mapping Antibiotics Resistance in Orchards. Washington Tree Fruit Research Commission Continuing Report.
Use pesticides with care. Apply them only to plants, animals, or sites listed on the labels. When mixing and applying pesticides, follow all label precautions to protect yourself and others around you. It is a violation of the law to disregard label directions. If pesticides are spilled on skin or clothing, remove clothing and wash skin thoroughly. Store pesticides in their original containers and keep them out of the reach of children, pets, and livestock.
YOU ARE REQUIRED BY LAW TO FOLLOW THE LABEL. It is a legal document. Always read the label before using any pesticide. You, the grower, are responsible for safe pesticide use. Trade (brand) names are provided for your reference only. No discrimination is intended, and other pesticides with the same active ingredient may be suitable. No endorsement is implied.
Important! Some of the pesticides discussed in this presentation were tested under an experimental use permit granted by WSDA. Application of a pesticide to a crop or site that is not on the label is a violation of pesticide law and may subject the applicator to civil penalties up to $7,500. In addition, such an application may also result in illegal residues that could subject the crop to seizure or embargo action by WSDA and/or the U.S. Food and Drug Administration. It is your responsibility to check the label before using the product to ensure lawful use and obtain all necessary permits in advance.
Treefruit.wsu.edu articles may only be republished with prior author permission © Washington State University. Republished articles with permission must include: “Originally published by Washington State Tree Fruit Extension Fruit Matters at treefruit.wsu.edu” along with author(s) name, and a link to the original article.




