by Elizabeth H. Beers and Erwin A. Elsner, originally published 1993
Typhlocyba pomaria McAtee (Homoptera: Cicadellidae)
The white apple leafhopper is the most common leafhopper found on apple in the Pacific Northwest. Until it became a pest of significance in Washington in the mid-1970s, specific control measures were rarely needed. Resistance to organophosphates is the most likely cause of increased populations. Since about 2000, it has again become an uncommon pest in most orchards, likely because of the shift away from pesticides toxic to its primary parasite.
White apple leafhopper is native to North America and occurs throughout the fruit growing areas of the United States and Canada, but its pest status varies from region to region.
Hosts
The white apple leafhopper attacks apple, cherry and prune but has also been found on peach and hawthorn. It does not usually damage pear, although the rose leafhopper has been noted in sizable numbers on this crop.
Life stages
Egg
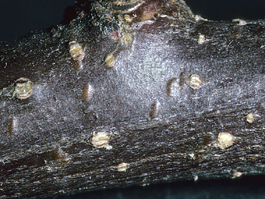
The egg is oblong and about 1/25 inch (1 mm) in length. The opaque embryo can be seen inside the developing egg. Overwintering eggs are inserted just beneath the bark of the host tree on 1- to 5-year-old wood, producing a crescent-shaped swelling in the bark. Eggs of the summer generation are inserted into leaf tissues and cannot be seen.
Nymph
The nymph is usually a translucent white color, although it occasionally can be bright yellow. It has thread-like antennae and, in the early instars, red eyes. The nymph grows from about 1/30 inch (0.8 mm) in the first instar to 1/10 inch (2.7 mm) in the fifth instar. The first two instars have no visible wingpad development, but wingpads become apparent in the last three instars. In the fifth instar, both pairs of wings can be distinguished in the wingpads.
Adult
The adults is 1/8 inch (3.4 mm) long, elongate, and pale yellowish white. The wings are held roof-like over the body.
Life history
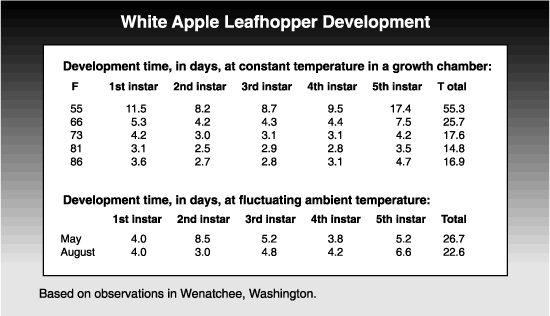
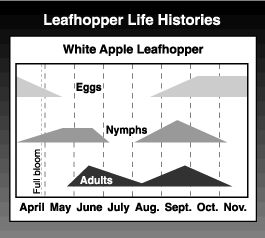 The overwintering eggs begin hatching at about the tight cluster stage of apple development (late March to mid-April), with peak hatch during or after bloom. There are five nymphal instars. It takes about 27 days for nymphs of the first generation to develop and 23 days for second generation nymphs.
The overwintering eggs begin hatching at about the tight cluster stage of apple development (late March to mid-April), with peak hatch during or after bloom. There are five nymphal instars. It takes about 27 days for nymphs of the first generation to develop and 23 days for second generation nymphs.
Adults begin to fly in late May and can be observed almost continuously from then until frost, although there are distinct peaks for the two generations. The first peak, in June, is typically much smaller than the second. After a 14-day preoviposition period, the adults mate, and the females deposit their eggs in the leaf tissue of the host tree, usually in the petiole, midrib or one of the larger veins. Adults live for several months. A females lays about 60 eggs over a long period. Second generation nymphs appear in mid- to late July. This generation is quite drawn out, with all stages of nymphs and adults overlapping. Small nymphs, from late-hatching eggs, can be found through harvest. Second generation adult activity increases sharply in late August and peaks from mid- to late September. Overwintering eggs are deposited during this period. Adults remain active until killed by a hard frost.
Damage

White apple leafhoppers have piercing-sucking mouthparts and cause damage to leaves by piercing mesophyll cells and removing their contents. The resulting damage appears as a white or yellowish-white stippling of the leaves. Leafhopper damage may superficially resemble mite feeding, but the individual spots are usually larger and there is no bronzing. Damage can be so extensive that injured leaves appear nearly white. Spur leaves in particular can be heavily damaged. Initially, feeding tends to be concentrated near the midrib, then eventually covers most of the interveinal spaces of the leaf blade. Leafhoppers generally prefer more mature leaves and will not infest shoots tips until the leaves have hardened off. Both nymphs and adults feed on leaves, but most damage is probably done by the nymphs.
Like other types of foliar damage, that caused by leafhoppers may reduce leaf photosynthesis, which in turn can affect the tree’s ability to set, size or mature a crop of fruit. However, no effect on fruit size or fruit quality has been found under Washington conditions, even when extremely high leafhopper populations were present. In addition, no reduction in return bloom or set was noted following a single season of heavy damage with a peak of 3 nymphs per leaf, although only one cultivar, Oregon Spur Red Delicious, has been studied. It is assumed that multiple seasons’ damage could eventually deplete the tree’s reserves, making effects of feeding damage more apparent, but this has not been studied. As with other indirect pest damage, factors such as tree vigor, tree age, drought stress, and damage by multiple pests could exacerbate the effect of leafhopper damage.
A second type of damage is the droplets of excrement, known as tar spots, which the leafhoppers deposit on leaves and fruit. Spotting of the leaves has never been considered important, but spotting of fruit is occasionally a concern. Informal tests indicate that tar spots are removed during the normal washing and brushing that precedes commercial packing of fruit, with the exception perhaps of the stem cavity, where brushes do not reach. However, spotting in the stem cavity is not very conspicuous (especially on darker skinned cultivars) and has not been reported to cause downgrading of fruit.
A third concern with leafhoppers has to do with the annoyance of fruit harvesters by high populations of adults. The harvest season for several of the major fruit varieties falls within the peak adult flight of the second generation. Adult leafhoppers jump and fly when disturbed and often get in the eyes, ears, nose, and mouth of people working close to the trees. When populations are high, the clicking of thousands of leafhoppers taking off is clearly audible.
Monitoring
All stages except the eggs of leafhoppers are easily monitored. Nymphs may be monitored by examining the leaves. Counting the nymphs on 20 leaves of 10 randomly selected trees per block should give an adequate assessment of leafhopper populations. Nymphs are normally concentrated on undersides of leaves, but both sides of the leaf should be examined. When the leaf is turned over to make the count, nymphs will tend to move to the other side, out of the sun, so counts should be done as rapidly as possible.
Nymphs may be counted at any time from tight cluster to frost, but typically sampling is done when a control decision must be made, which is at about petal fall for first generation nymphs and again in mid-August for second generation nymphs. Sampling when most of the leafhoppers are in the adult stage will tend to underestimate the population.
Adults may be monitored with a sticky trap, such as a yellow panel of the type used for cherry fruit fly. The unbaited version of this trap is preferable, since it is less attractive to flies of all types. Adults can also be caught on traps of various other colors, but yellow is one of the most attractive colors. Although useful in following population trends, adult monitoring will probably not play an important role in management decisions.
Overwintering eggs are more difficult to sample than active stages, but this can be done at any time from late October until eggs begin to hatch the following spring. A standard method is to take 4 inches (10 cm) of the current season’s growth from the bud scar toward the tip. Although eggs are laid in older wood, they become progressively more difficult to see as the wood gets rougher. Also, the scars of eggs from previous seasons can be mistaken for viable eggs on older wood, a problem which does not occur on the new growth. Sampling eggs may be useful in determining levels of parasitism but is unnecessarily laborious for management decisions. Nymphs may be sampled much more easily with enough lead time to apply control measures.
Biological control
The primary biological control agent of leafhoppers in the Pacific Northwest is an egg parasitoid, Anagrus sp. (probably A. epos), a mymarid wasp (see section on Anagrus). It attacks both overwintering and summer eggs of white apple leafhopper. In overwintering eggs, parasitism levels of up to 25% have been found in the Wenatchee area. The level of parasitism appears to be generally related to the intensity of pesticide use (primarily that for codling moth) the previous season, but no single application timing or material has been identified. Research in Michigan indicates that more than 90% of the overwintering generation of eggs can be parasitized in unsprayed orchards so broad-spectrum pesticides undoubtedly suppress parasitoid populations. Levels of parasitism found in conventionally managed orchards are generally too low to prevent substantial populations from developing. Anagrus could potentially play a role in biological control of leafhoppers if broad-spectrum chemicals are reduced or removed from the spray program.
A second parasitoid is a wasp in the family Dryinidae (probably Aphelopus sp.), which is a parasitoid of both nymphs and adults. It develops internally in leafhopper nymphs, then appears on the adults as a pouch on the abdomen. This parasitoid has been collected from cherry and apple orchards in the mid-Columbia area of Oregon, although it may be much more widely distributed. While substantial rates of parasitism have been reported, the potential of this parasitoid species as a biological control agent is unknown. Despite extensive examination of leafhoppers in Washington, this parasitoid species has not been found.
Management
Because leafhoppers are indirect pests, they should only be controlled when necessary. It is difficult to set treatment thresholds for white apple leafhopper since no fruit size, fruit quality, or return bloom reduction due to leafhopper damage has been observed. Other factors must be taken into account when deciding whether control is needed. Since the major form of biological control occurs during the egg stage, this mortality is already accounted for when nymphal populations are assessed. If the trees are very young, excessive damage to the relatively small leaf canopy could retard growth. In vigorous, mature trees with a high leaf-to-fruit ratio, moderate to heavy leafhopper damage will probably have little effect. Similarly, if the crop is very light, more foliar damage can probably be tolerated than if the crop load is heavy. The potential for drought because of light soils, inadequate irrigation schedules, or extremely hot, dry weather could also serve as a reason to consider controlling leafhoppers or other indirect pests. If substantial foliar damage by mites or leafminers has already been incurred, further damage by leafhoppers should probably be avoided.
White apple leafhoppers appear to have some resistance to the organophosphate insecticides commonly used in orchards. Tests at Wenatchee have shown up to 75% reduction of nymphal populations, depending on the material. Although most orchardists have come to expect much higher levels of control, this may be adequate to keep populations at a tolerable level. There also is some evidence of resistance to organochlorine insecticides, which varies from region to region, but carbamate insecticides still appear to be very effective in most areas. Insecticidal soaps, which are relatively new products in commercial orchards, are moderately effective against leafhoppers and may be a tool for resistance management. In addition, these materials should have minimal impact on beneficials because of their short residual activity. The development of resistance to organophosphate insecticides indicates that resistance management should be of concern in the future.
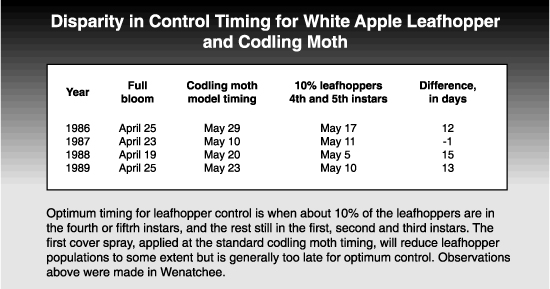
Optimum timing for control of the first generation is when most of the overwintering eggs have hatched but before the majority of the nymphs are in the last two instars, when they are harder to kill. A somewhat arbitrary target timing for this point in the leafhopper’s phenology is when about 10% of the population is in the fourth and fifth instars, with 90% still in the first, second, and third instars. This is usually at or slightly after petal fall. The first cover spray, applied at the standard codling moth timing, will also reduce leafhopper populations somewhat, as will carbaryl when used as a fruit thinning material. In general, however, first cover timing is too late for optimum leafhopper control.
Control of the first generation is generally easier to achieve than control of the second generation, since the most susceptible stages are present at a specific period and sprays can be targeted more precisely. An exception to the strategy outlined above is the use of insecticidal soaps. These materials appear to kill all nymphal instars equally well. Their residual activity is very short (probably only direct spray contact is effective) so egg hatch should be complete before application. For these reasons, timing will be slightly later than for conventional insecticides. Control of first generation nymphs with soap has been fairly successful, but control of the second generation has not.
Second generation white apple leafhopper populations can greatly exceed the first, often surprising producers and consultants by the size of a late season infestation. Adults are quite mobile, and during the first generation flight they can move into and rapidly reinfest a block that was treated for first generation nymphs. If a clean orchard is surrounded by infested ones, then second generation nymphs should be sampled. Because of a prolonged egg hatch in the second generation, multiple stages occur at any one time, making control of this generation less likely to be satisfactory than that of the first generation. However, if picker annoyance reduction is the primary concern, then it may be more appropriate to control this generation. Insecticide applications should be made before adult populations begin to rise sharply, which usually occurs in mid- to late August.
As leafhoppers occur largely on the undersides of the leaves and are well distributed throughout the canopy, spray coverage should be thorough to get adequate control. Limited tests using aerial applications have shown this method to be considerably less satisfactory than ground application. No information is available on the effect of concentrate versus dilute sprays although, assuming good coverage, both methods should be adequate.