Updated by Tianna DuPont, WSU Extension; Louis Nottingham, Robert Orpet, WSU Entomology, Rick Hilton OSU. May 2022. Adapted from by Everett C. Burts, Helmut Riedl, and John Dunley, originally published 1993. FS376E_Pear Psylla Integrated Pest Management
Introduction
Pear psylla (Cacopsylla pyricola [Foerster] [Hemiptera: Psyllidae]) is an important pest of pear in Washington. Honeydew produced by pear psylla causes fruit russet, and serious infestations can stunt and defoliate trees.
History
Pear psylla likely arrived in the United States along with shipments of pear nursery stock from western Europe. It was first found in Connecticut in 1832 and spread to Washington State by 1939 (Westigard et al. 1979). Within a few years it became a serious pest throughout all pear growing areas in the Pacific Northwest.
Hosts
In the Pacific Northwest, pear psylla is a pest only of pear. Several other plants are transitory hosts and overwintering sites for winterform pear psylla adults. Pear psylla adults may feed on other deciduous fruit trees including apples, conifers and shrubs as they disperse from pear orchards in the fall and return in the spring (Horton et al. 1994; Cooper et al. 2019). However, pear psylla does not reproduce on these transitory hosts (Kaloostian 1970; Cooper et al. 2019).
Life stages
Egg
The egg, shaped like a grain of rice, is attached to the host by a small protrusion extending from the rounded end and inserted into host tissue. The egg is creamy white when laid but turns yellow to orange as it develops (Figure 1).
Nymph
The nymph passes through five stages (instars). The first instar is translucent yellow. It is somewhat cylindrical in shape and about the size of the egg. Each successive instar is larger, flatter and more oval than the previous instar. The fourth instar nymph is yellowish green to light tan. The fifth instar is dark green to dark brown and often referred to as the hardshell stage. Wing pads are noticeable on third instars and get progressively larger on fourth and fifth instars (Figures 1–3).
Adult
There are two adult forms: winterform and summerform. Both forms of adults hold their wings roof-like over the abdomen. Adults have reddish brown bodies with black markings, and winterforms may appear almost black. The winterform is larger (wing length 2.3 to 2.5 mm) than the summerform (wing length 1.6 to 1.8 mm) (Figures 4 and 5) (Slingerland 1892; Wong and Madsen 1967; Burckhardt and Hodkinson 1986).
Life History
Pear psylla overwinter as winterform adults in a state of reproductive diapause. They begin laying eggs when pear buds begin to swell. First, eggs are deposited on the wood, generally at the base of fruit and leaf buds (Horton 1999). Offspring of the overwintered generation become summerform adults first appearing in mid-May. Pear psylla has two to four summerform generations in most pear-growing regions, with generally two complete summerform generations occurring in Washington (Horton 1999). Summerform adults tend to lay eggs on rapidly growing leaf tissues, often placing eggs along the leaf mid-vein (Horton 1990).
Damage
Fruit russet
Nymphs and adults are phloem feeders. Honeydew, produced by nymphs, drips or runs onto fruit, causing dark, russet blotches or streaks and downgraded fruit (Figure 6). The damage may be exacerbated by a sooty mold fungus that colonizes the honeydew and also marks fruit (Burts 1970).
Psylla shock
In large numbers, pear psylla can stunt and defoliate trees and cause fruit drop. A carryover effect may reduce fruit set the following year. These symptoms, called psylla shock, are caused by toxic saliva from feeding nymphs (Westigard et al. 1979).
Decline
Pear psylla also transmit a mycoplasma disease organism (Candidatus Phytoplasma pyri: Pear decline phytoplasma) through its saliva. The disease damages sieve tubes in the phloem. This damage prevents nutrients from moving down the tree and results in root starvation. Trees grafted on Ussurian pear (P. ussuriensis) and Asian pear (P. pyrifolia, synonymous with P. serotina) rootstocks are the most susceptible. Trees grafted on P. communis, P. betulifolia, P. calleryana, and Cydonia oblongata (quince) rootstocks become infected but are tolerant and display reduced decline symptoms (Blomquist and Kirkpatrick 2002; Teng et al. 2002). Most pears in Washington and Oregon are grafted to tolerant P. communis (Elkins et al. 2012).
Monitoring
Weekly monitoring is recommended.
Adults
Monitor adults with beat tray sampling (Figure 7). Hold an 18-inch square tray with a white cloth cover one foot below a 0.75 to 1.5-inch diameter limb with an average number of spurs and branches. Tap the limb firmly three times with a stiff rubber hose. Count adults jarred from the limb onto the tray. Thirty trays at random through the sampling area is standard for a pear block of ten to twenty acres.
Nymphs and eggs
To determine the density of first-generation eggs and nymphs, examine spurs. Collect ten fruiting spurs with 0.5 to 2 inches of wood. Count eggs on the wood and count eggs and nymphs on emerging green tissue (once present) with a dissecting microscope or a ten-power hand lens.
Subsequent generations of eggs and nymphs should be sampled on new shoot growth. Collect a total of ten leaves from each of ten randomly selected trees. Select five leaves from the lower canopy with two in the center of the canopy near the crotch of the scaffold limbs and three in the middle of each of two scaffold limbs (Figure 8). Include one to two leaves that may not receive good coverage in the center of the tree. Use a telescopic pruner to collect five leaves across two clusters or shoots in areas which are difficult to spray, such as the upper canopy and the back side of limbs.
Biological control
Important biological control organisms in Washington pear orchards are the parasitic wasp Trechnites insidiosus; true bugs Deraeocoris brevis, Campylomma verbasci, and Anthocoris spp.; lacewings Chrysoperla carnea, Chrysopa nigricornis, Hemerobius spp.; and the earwig Forficula auricularia.
Trechnites insidiosus
Trechnites insidiosus can parasitize pear psylla at rates that exceed 70% in unsprayed orchards and 50% in organic orchards (Beers et al. 1993). Maximum parasitism rates in a review of nineteen field studies range from 1.7% to 100% (Tougeron et al. 2021). Over half of the studies reported parasitism rates exceeding 40% (Tougeron et al. 2021). Trechnites insidiosus generally has four generations per year in Washington orchards. Peak counts may often occur at approximately bloom time as parasitoids emerge from their overwintering sites within psylla hosts (Figures 9 and 10).
Deraeocoris brevis
Deraeocoris brevis is an abundant predator found in Pacific Northwest apple and pear orchards. Deraeocoris brevis may consume approximately 200 psylla eggs per day (Booth 1992). Overwintering adults can be found active in orchards starting in early March. Egg lay begins in April or May, and first-generation nymphs generally occur starting in mid-May (Yakima) to early June (Wenatchee) (Horton et al. 2012; DuPont and Strohm 2020). Deraeocoris brevis has two to three generations per year in Washington and is present from April to October (DuPont and Strohm 2020) (Figures 11 and 12).
Anthocoris
Anthocoris spp. are close relatives of the minute pirate bug (Orius) and comprise a mixture of two or three species in Pacific Northwest orchards. They are well adapted to feed on pear psylla and can play a major role in the biological control of this pest. Anthocoris spp. are found occasionally in Wenatchee River Valley orchards and commonly in the Yakima Valley in Washington (Horton and Lewis 2000; Horton et al. 2012; Horton and Lewis 2014). They overwinter as adults in multiple habitats, have multiple generations per year, and are frequently active very early. They have a strong preference for psyllids and are common outside of orchards, often occurring on willow, alder, poplar, and bitterbrush, among other trees and shrubs (Horton and Lewis 2000).
Campylomma verbasci
Campylomma verbasci is a known pest in apple, but it is a beneficial predator of pear psylla and is not known to cause economic injury in pears. Campylomma can consume more than 170 pear psylla eggs per day in the laboratory (Booth 1992). Campylomma also consume young pear psylla nymphs and hardshells, eating an average of four eggs, five young psylla nymphs, and two hardshells per day in one study (Nelson 1985). Campylomma overwinters in the egg stage and has three generations per year in Washington (DuPont and Strohm 2020) (Figures 13 and 14).
European earwig
European earwig (Forficula auricularia) is an important predator in pear and apple orchards, feeding on aphids, pear psylla, mites, and insect eggs. In one study, young earwigs consumed as many as 1,000 pear psylla eggs per day (Lenfant et al. 1994). Earwigs overwinter and rear their young in nests underground. They are found in the orchard canopy at night beginning in June in central Washington (Figures 15 and 16).
Lacewings
Brown lacewings (Hemerobius spp.) occur sporadically in central Washington pear orchards and are most abundant from July until late September (Horton et al. 2012) (Figure 17).
Green lacewings (Chrysoperla carnea/plorabunda, Chrysopa nigricornis) are predators of aphids and, to a lesser extent, psyllids (Carroll and Hoyt 1984). Chrysoperla carnea adults are generally seen earlier in Washington orchards (first seen in April) than C. nigricornis (starting mid-May to July) (Horton et al. 2012; DuPont and Strohm 2020) (Figures 18 and 19). Immatures are more common starting in May in Yakima or June in Wenatchee. They are active until September (Horton et al. 2012; DuPont and Strohm 2020). Green lacewings are common outside of orchards on many woody and herbaceous plants.
Thresholds
An important aspect of integrated pest management is the use of economic thresholds (ET) to make spray decisions. Control is recommended at an ET where pest densities are projected to surpass an economic injury level (EIL), the minimum pest density where the economic loss due to the pest is equal to the cost of controlling the pest (Higley and Pedigo 1996).
Research has identified the quantity of fruit damage we might expect at varying levels of pear psylla pest densities. DuPont et al. 2023 found >2% culls (US grade 3) occur with >0.4 second or third generation pear psylla nymphs per leaf, >0.6 second generation pear psylla adults per tray, or >1.1 third generation pear psylla adults per tray (Figures 20 and 21). This is similar to previous studies. Burts (1988) reports >0.3 pear psylla nymphs per leaf results in detectable fruit russet (Burts 1988). Westigard et al. (1981) report 5% fruit downgrades occur with 2 pear psylla nymphs per leaf for Bosc or 0.4 nymphs per leaf for Anjou.
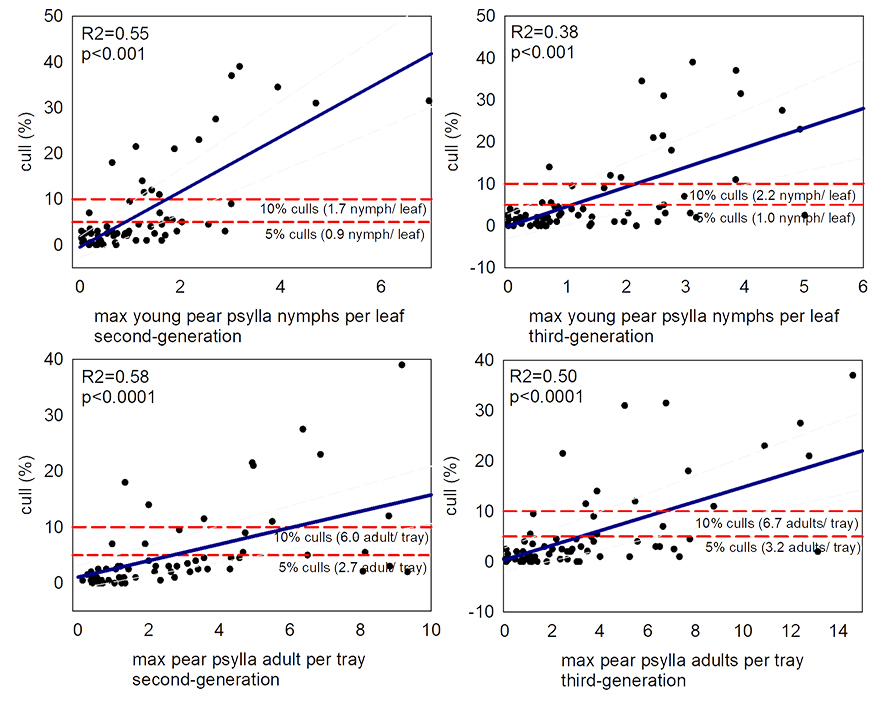
To avoid fruit damage, managers should spray before pear psylla reach injurious levels. In pear psylla management prebloom sprays are essential. During the second and third generation managers can modify spray intensity, depending on whether pear psylla are predicted to exceed economic thresholds.
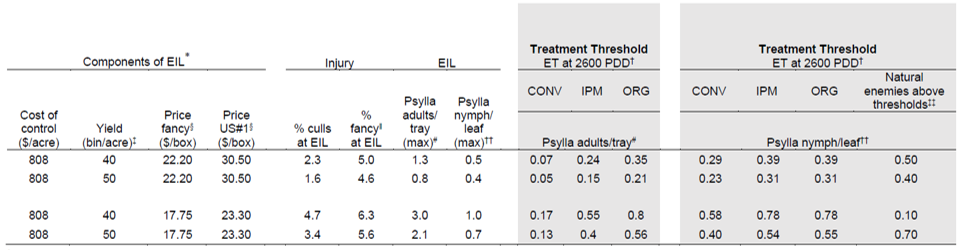
To determine what economic threshold to use, managers must consider the value of losses, the economic cost of management, the delay between monitoring and management, and prospects for biocontrol. The EIL will vary depending on yield and price. To use Tables 1 and 2:
- Select yield and pricing which most closely align with your system.
- Identify the economic injury level that coincides with your scenario.
- Use the corresponding Treatment Threshold to determine whether pear psylla populations are likely to reach economically damaging levels if not controlled.
- If natural enemies are above biological control thresholds in the third generation (shown in Table 3) use the higher Treatment Thresholds. Natural enemy abundance is predicted to slow or prevent population growth of pear psylla (Table 3).

*The economic injury level is set to where the cost of downgrades equals the cost of management, assuming an average management cost of $808 per acre. Management costs include five critical sprays using 2022 prices and a spray application labor cost of $36 per hour. Spray applications include: kaolin at 75 PDD (dormant), kaolin+pyriproxyfen+oil at 200 PDD (bud burst), kaolin+pyriproxyfen at 350 PDD (popcorn), kaolin+spirotetromat at 900 PDD, and kaolin+spirotetramat+oil at 1200 PDD. Additional sprays at 1500 and 2600–3200 PDD should depend on economic thresholds.
†Using population growth models to predict population at 1300 PDD the spray timing (1400–1750 PDD) to target generation pear psylla which coincides with maximum populations at the EIL.
‡1,100 lb bin.
§Free on board (FOB) prices from Pear Marketing Association for size class 90 where the high price example is the average from the 2020/21 year and low prices are the average from the 2018/19 year. 44 lb box. Revenue assumes price minus $13.50 per box packing charges.
ǁFancy estimates based on estimated downgrades at designated psylla nymph levels.
#Average of 30 beat tray samples per 10 acres.
††Average of 100 leaves per 10-acre orchard.
*The economic injury level is set to where the cost of downgrades equals the cost of management assuming an average management cost of $808 per acre. Management costs include five critical sprays using 2022 prices and spray application labor cost of $36 per hour. Spray applications include: kaolin at 75 PDD (dormant), kaolin+pyriproxyfen+oil at 200 PDD (bud burst), kaolin+pyriproxyfen at 350 PDD (popcorn), kaolin+spirotetromat at 900 PDD, and kaolin+spirotetramat+oil at 1200 PDD. Additional sprays at 1500 and 2600–3200 PDD should depend on economic thresholds. Assuming a 50% reduction in pest population per spray.
†Using population growth models to predict the population at 2600 PDD the spray timing (2800–3200 PDD) to target third generation pear psylla before young nymphs molt into hardshells which coincides with maximum populations at the EIL. Assumes EIL at 4000 PDD (harvest timing).
‡1,100 lb bin.
§Free on board (FOB) prices from Pear Marketing Association for size class 90 where the high price example is from the 2020/21 year and low prices are from the 2018/19 year. Revenue assumes price minus $13.50 per box packing charges.
ǁFancy estimates based on estimated downgrades at designated psylla nymph levels (regressions Table 2).
#Average of 30 beat tray samples per 10 acres.
††Average of 100 leaves per 10-acre orchard.
‡‡D. brevis immatures exceed 6 per 30 trays, C. verbasci immatures exceed 3 per 30 trays, or earwig populations exceed 1.5 per 30 trays or 2 per trap.
Table 3: Natural densities above biological control thresholds
| Insect Type | Insects per Beat Tray | Insects per 30 Beat Trays | Insects per Trap |
|---|---|---|---|
| D. brevis immatures | 0.2 | 6 | |
| C. verbasci immatures | 0.1 | 3 | |
| European earwigs | 0.05 | 1.5 | 2 |
Cultural Tactics
Summer Pruning
The removal of vegetative shoots from trees is an important cultural control. Summer pruning improves spray penetration and light in the canopy. If timed correctly, pruning can also reduce the pear psylla population and amount of honeydew in trees. Prune between 2100–2400 PDD to remove nymphs before they molt into third generation adults (Figure 22).

Honeydew Washing
Washing honeydew off fruit trees with overhead sprinklers or airblast sprayers can significantly reduce fruit marking damage (Brunner and Burts 1981). Honeydew washing methods differ from overhead irrigation and are only used to remove honeydew. Under-tree sprinklers are recommended for general irrigation to reduce disease risk and increase irrigation efficiency. It is critical to limit honeydew washes, because washing too often and for too long can cause disease issues. Time washing to target honeydew from old nymphs of the second and third generations at 1600–2400 PDD and 3500–4000 PDD, respectively. Washing is not necessary if visible honeydew is not apparent. In replicated on-farm trials, one to two washes with systems of 60–80 GPM per acre for eight to twelve hours effectively reduces fruit marking (Strohm and DuPont, unpublished data). For airblast sprayer washes, use at least 800 GPA for smaller trees, and increase gallonage with tree size.
Chemical Tactics
Pre-bloom applications
Dormant/Delayed Dormant (75–100 PDD)
A particle film application (Surround CF/WP or Celite 610 at 50 lb/acre) should be made as early as it is safe to drive a sprayer through the orchard. This spray prevents pear psylla from colonizing the orchard. Particle films reduce pear psylla adult colonization and egg lay by 80–100%, which reduces pear psylla pressure for the first generation (Hull et al. 2008; Nottingham et al. 2020; Nottingham and Beers 2022) (Figure 22). In some years, a repeat application may be necessary during delayed dormant after a rain or if many weeks have passed since the first application. Effectiveness and longevity of particle films is improved when combined with a spreader sticker. A sulfur or lime sulfur application with oil can also suppress pear rust mites and spider mites in addition to pear psylla adults.
Budburst (200 PDD)
Pear psylla adults will begin laying eggs on soft green tissues as soon as they emerge from flower buds. In some years and orchards, budburst is the earliest growers are able to spray due to wet ground. Applications just before budburst help prevent pear psylla adults from laying eggs on freshly emerging bud tissues. A second particle film (Surround CF or Celite 610 at 50 lb/acre) applied just before budburst renews particle film residues, repels pear psylla adults, and prevents egg lay. If budburst is the first spray a grower can make, a second particle film spray at popcorn may be necessary. Pyriproxyfen (Esteem 35WP) is an insect growth regulator that can sterilize pear psylla adults and has little nontarget effect on natural enemies (Higbee et al. 1995; Dunley et al. 2001; Nottingham and Beers 2022). If greater suppression is needed, pyriproxyfen can be mixed with other nondisruptive materials, such as diflubenzuron (Dimilin 2L), buprofezin (Centaur WDG), cinnamon oil (Cinnerate), or azadirachtin (Aza-Direct or Neemix 4.5) (Nottingham et al. 2019; Nottingham and Sater 2021b).
Popcorn (350 PDD)
The insect growth regulator pyriproxyfen (Esteem 35WP) sprayed at popcorn will sterilize pear psylla adults and have little negative effect on natural enemies (Dunley et al. 2001). If the 14-day window required between applications of pyriproxyfen has not been met, other selective materials such as cinnamon oil (Cinnerate), azadirachtin (Aza-Direct or Neemix 4.5), diflubenzuron (Dimilin 2L), or buprofezin (Centaur WDG) can be used instead (Nottingham and Sater 2021a). If pear psylla adult pressure is high, a particle film (Surround CF or Celite 610) sprayed just before bloom renews the residue to repel pear psylla adults from trees.
Post-bloom conventional applications
Prior to summerform adult and young nymph emergence (900 DD)
Apply a particle film (e.g., Surround CF, Celite 610 at 50 lb/acre) at 900 PDD to deter the emerging summerform adults from landing on trees and laying eggs. Application of spirotetramat (Ultor or Movento) at this timing will reduce survival of nymphs as they hatch (Wise et al. 2008; Beers and Greenfield 2014; Wise et al. 2018). This material works best when two applications are made, so a second application can be made in 14 days, at approximately 1200 PDD. Spirotetramat can only be applied twice per season, and applications must be at least 14 days apart.
Prior to peak adults and eggs (1200 PDD)
At 1200 PDD (at least two weeks after the previous spray), applying a second particle film repels summerform adults from trees, and a second spirotetramat application (Ultor or Movento) suppresses newly hatched nymphs. If this is the first spirotetramat application, a second can be made in 14 days, at approximately 1500 PDD.
Prior to peak young nymphs second generation (1500 PDD)
If only one particle film (Surround WP or Celite 610 at 50 lb/acre) was applied after bloom, a second (final) application at 1500 PDD will repel the remaining summerform adults, preventing further egg lay. No more than two particle film applications should be made after bloom, as this can increase the risk of mite outbreaks. If only one application of spirotetramat (Ultor or Movento) has been made and it has been 14 or more days, a second application at this timing targets young nymphs. If pressure is high in the summer, additional sprays with more toxic materials are warranted (see Tables 1 to 3), the most effective time to make insecticide applications is as young nymphs are increasing toward peak and prior to hardshells (1400–1750 PDD). Use products that kill young nymphs, such as diflubenzuron (Dimilin 2L), cinnamon oil (Cinnerate), or azadirachtin (Aza-Direct or Neemix 4.5). Do not use azadirachtin products on Comice pears.
Third generation pear psylla (2600–3200 PDD)
Time insecticides to 2600–3200 PDD, before young nymphs molt into hardshells. Hardshells are harder to kill, and once they are present (starting 3000–3400 PDD) the optimal spray timing has passed. Consider pear psylla and natural enemy thresholds to determine the necessity for sprays for third generation pear psylla. If pear psylla are projected to surpass damage thresholds, an insect growth regulator, such as pyriproxyfen (Esteem 35WP) or diflubenzuron (Dimilin 2L), will suppress this generation of pear psylla. Alternatively, the organic insecticides cinnamon oil (Cinnerate) or azadirachtin (Aza-Direct or Neemix 4.5) can also suppress pear psylla. Do not use azadirachtin products on Comice pears.
Postbloom organic applications
For organic sprays, scout and begin spraying organic insecticides weekly once young nymphs become present, and continue until just past young nymph peak, 1100–1800 PDD (second generation) and 2800–3500 PDD (third generation). Consider pear psylla and natural enemy thresholds to determine the necessity for sprays at this timing. Products to use include summer oil, Cinnerate, or neem (Aza-Direct, Neemix, or Rango) (Nottingham and Sater 2021b). Do not use azadirachtin products on Comice pears. If pear psylla numbers are high, these products can be mixed to improve efficacy, but extra care should be taken to avoid marking or phytotoxicity.
Selective Codling Moth and Mite Management
For successful pear psylla integrated pest management, codling moth and mite sprays need to be compatible with pear psylla biological control. Include effective materials with few indirect effects on natural enemies.
If spider mites are found, give predators time to suppress them. In areas where pear rust mites or spider mites are becoming a problem, selective miticides such as fenbutatin (Vendex 50WP), spirodiclofen (Envidor 2SC), or cyflumetofen (Nealta) are effective and have relatively low indirect impacts on natural enemies.
Codling moth management should include mating disruption and effective use of selective pesticides. The first codling moth spray targets eggs and should be conducted at the standard (225–275 codling moth degree day [DD]) or delayed first cover (375 DD) timing. This spray can include materials such as oil and methoxyfenozide (Intrepid 2F). The second spray should be conducted at 425 DD (standard timing) or 525 DD (delayed first cover timing) and can include oil in addition to larvicides such as granulovirus (Cyd-X HP) or diflubenzuron (Dimilin 2L). For second and third generations of codling moth, add 1000 DD to the previous timings, but treatment may not be necessary. For more information see Codling Moth.
Materials Available for Pear Psylla
Excerpt from the WSU Crop Protection Guide. For timings at which each pesticide can be used refer to the Crop Protection Guide.
Materials available for pear
Excerpt from the WSU Crop Protection Guide. For timings at which each pesticide can be used refer to the Crop Protection Guide.
Acknowledgements
Thank you to funding support from the Washington State Department of Agriculture Specialty Crop Block Grant program, the USDA Crop Protection Grant program, the Washington State Tree Fruit Research Commission and the Fresh and Processed Pear Committee.
Contacts
Robert Orpet
Research Assistant Professor
WSU Tree Fruit Research & Extension Center
1100 N Western Ave., Wenatchee, WA 98801
robert.orpet@wsu.edu
Tianna DuPont
Tree Fruit Extension Specialist
Washington State University
tianna.dupont@wsu.edu
(509) 293-8758
(509) 713-5346 (cell)
References
Beers, E., J. Brunner, M. Willett, and G. Warner. 1993. Orchard Pest Management. Good Fruit Grower.
Beers, E.H., and B.M. Greenfield. 2014. Pear Psylla Insecticide Test, 2013. Arthropod Management Tests 39(1).
Blomquist, C.L., and B.C. Kirkpatrick. 2002. Frequency and Seasonal Distribution of Pear Psylla Infected with the Pear Decline Phytoplasma in California Pear Orchards. Phytopathology 92(11):1218–1226.
Booth, S.R. 1992. The Potential of Endemic Natural Enemies to Suppress Pear Psylla, Cacopsylla pyricola Förster, in the Hood River Valley, Oregon.
Brunner, J.F., and E. Burts. 1981. Potential of Tree Washes as a Management Tactic Against Pear Psylla. Journal of Economic Entomology 74:71–74.
Burckhardt, D., and I.D. Hodkinson. 1986. A Revision of the West Palaearctic Pear Psyllids (Hemiptera: Psyllidae). Bulletin of Entomological Research 76(1):119–132.
Burts, E.C. 1970. Pear Psylla in Central Washington. Washington State University.
Burts, E.C., 1988. Damage threshold for pear psylla nymphs (Homoptera: Psyllidae). Journal of economic entomology 81, 599-601.
Carroll, D.P., and S.C. Hoyt. 1984. Natural Enemies and Their Effects on Apple Aphid, Aphis pomi Degeer (Homoptera, Aphididae), Colonies on Young Apple Trees in Central Washington. Environmental Entomology 13(2):469–481.
Cooper, W.R., D.R. Horton, M.R. Wildung, A.S. Jensen, J. Thinakaran, D. Rendon, L.B. Nottingham, et al. 2019. Host and Non-host “Whistle Stops” for Psyllids: Molecular Gut Content Analysis by High-Throughput Sequencing Reveals Landscape-Level Movements of Psylloidea (Hemiptera). Environmental Entomology 48(3):554–566.
Dunley, J.E., B.M. Greenfield, G.T. Hannig, and L.H. Bennett. 2001. Control of Pear Psylla with Diflubenzuron and Pyriproxyfen, 2000. Arthropod Management Tests 26(1):A33.
DuPont, S.T., and C.J. Strohm. 2020. Integrated Pest Management Programmes Increase Natural Enemies of Pear Psylla in Central Washington Pear Orchards. Journal of Applied Entomology 144(1–2):109–122.
DuPont, S.T., C. Strohm, L. Nottingham, R. Hilton, and R. Orpet. n.d. Psylla and Natural Enemy Thresholds for Successful Integrated Pest Management in Pears. Washington State University.
Elkins, R., R. Bell, and T. Einhorn. 2012. Needs Assessment for Future US Pear Rootstock Research Directions Based on the Current State of Pear Production and Rootstock Research. Journal of American Pomological Society 66(3):153–163.
Higbee, B.S., D.R. Horton, and J.L. Krysan. 1995. Reduction of Egg Hatch in Pear Psylla (Homoptera, Psyllidae) after Contact by Adults with Insect Growth Regulators. Journal of Economic Entomology 88(5):1420–1424.
Higley, L.G., and L.P. Pedigo. 1996. The EIL Concept. In Economic Thresholds for Integrated Pest Management, L.G. Higley and L.P. Pedigo (eds.), 9–36. University of Nebraska Press, Lincoln.
Horton, D.R. 1990. Distribution and Survival of Eggs of Summerform Pear Psylla (Homoptera: Psyllidae) Affected by Leaf Midvein. Environmental Entomology 19(3):656–661.
Horton, D.R. 1999. Monitoring of Pear Psylla for Pest Management Decisions and Research. Integrated Pest Management Reviews 4:1–20.
Horton, D.R., E.C. Burts, T.R. Unruh, J. Lunar, K. Krysan, L.B. Coop, and B.A. Croft. 1994. Phenology of Fall Dispersal by Winterform Pear Psylla (Homoptera: Psyllidae) in Relation to Leaf Fall and Weather. The Canadian Entomologist 126(1):111–120.
Horton, D.R., and T.M. Lewis. 2000. Seasonal Distribution of Anthocoris spp. and Deraeocoris brevis (Heteroptera: Anthocoridae, Miridae) in Orchard and Non-orchard Habitats of North Central Washington. Annals of the Entomological Society of America 93(3):476–485.
Horton, D.R., and T.M. Lewis. 2014. Seasonal Distribution of Anthocoris spp. and Deraeocoris brevis (Heteroptera: Anthocoridae, Miridae) in Orchard and Non-orchard Habitats of Central Washington. Annals of the Entomological Society of America 93(3):476-485.
Horton, D.R., E.R. Miliczky, V.P. Jones, C.C. Baker, and T.R. Unruh. 2012. Diversity and Phenology of the Generalist Predator Community in Apple Orchards of Central Washington State (Insecta, Araneae). The Canadian Entomologist 144(5):691–710.
Hull, L.A., F.U. Zaman, and K.J. Neelendra. 2008. Control of Seasonal Pear Psylla Populations in Pears. Arthropod Management Tests 34:A23.
Jones, V.P. 2020. How to Effectively Manage Codling Moth. Washington State University Tree Fruit. Washington State University.
Kaloostian, G.H. 1970. Transitory Hosts of the Pear Psylla. Journal Economic Entomology 63(4):1039–1041.
Lenfant, C., A. Lyoussoufi, X. Chen, F.F. Darcier, and B. Sauphanor. 1994. Potential of Forficula auricularia L. as Predator of Pear Psylla Cacopsylla pyri (L.). Entomologia Experimentalis et Applicata 73(1):51–60.
Nelson, B.A. 1985. Campyloneura virgula (Herrick-Schaffer) (Hemiptera: Miridae): Biology, Tolerance to Selected Insecticides and Potential as a Predator of Pear Psylla, Psylla pyricola Foerster (Homoptera: Psyllidae). Department of Entomology. Washington State University.
Nottingham, L., and E.H. Beers. 2022. Improving Pear Pest Management with Integrated Approaches. Washington State Tree Fruit Research Commission, Wenatchee, WA.
Nottingham, L.B., R. Orpet, and E. Beers. 2020. Greenhouse Test on Repellents of Winterform Pear Psylla. Arthropod Management Tests 45(1).
Nottingham, L.B., R. Orpet, B. Greenfield, and E. Beers. 2019. Chemical Control of Pear Psylla in Pear, 2018. Arthropod Management Tests 44(1):1–2.
Nottingham, L., and C. Sater. 2021a. Laboratory Bioassay of Organic Insecticides on Pear Psylla, 2020. Arthropod Management Tests 46(1).
Nottingham, L., and C. Sater. 2021b. Organic and Conventional Insecticide Programs for Control of Pear Psylla in Pear, 2020. Arthropod Management Tests 46(1).
Slingerland, M.V. 1892. The Pear-Tree Psylla. Psylla pyricola. Cornell University Agricultural Experiment Station Entomological Division.
Teng, Y.W., K. Tanabe, and A. Itai. 2002. Genetic Relationships of Pyrus Species and Cultivars Native to East Asia Revealed by Randomly Amplified Polymorphic DNA Markers. Journal of the American Society for Horticultural Science 127(2):262–270.
Tougeron, K., C. Iltis, F. Renoz, L. Albittar, T. Hance, S. Demeter, and G.J. Le Goff. 2021. Ecology and Biology of the Parasitoid Trechnites insidiosus and Its Potential for Biological Control of Pear Psyllids. Pest Management Science 77(11):4836–4847.
Westigard, P.H., P.B. Lombard, and D.W. Berry. 1979. Integrated Pest Management of Insects and Mites Attacking Pears in Southern Oregon. Oregon State University Agricultural Experiment Station Publication Station Bulletin 634. Oregon State University.
Westigard, P.H., R.B. Allen, and L.J. Gut. 1981. Pear Psylla: Relationship of Early-Season Nymph Densities to Honeydew-Induced Fruit Damage on Two Pear Cultivars. Journal of Economic Entomology 74(5):532–534.
Wise, J.C., R. Vander Poppen, and L.J. Gut. 2008. Control of Pear Psylla, 2007. Arthropod Management Tests 33:A25.
Wise, J.C., A.H. Van Woerkom, C.E. Wheeler, and L.J. Gut. 2018. Control of Pear Psylla in Pear, 2017. Arthropod Management Tests 43(1):tsy062.
Wong, T.T.Y., and H.F. Madsen. 1967. Laboratory and Field Studies on the Seasonal Forms of Pear Psylla in Northern California. Journal of Economic Entomology 60(1):163–168.
Use pesticides with care. Apply them only to plants, animals, or sites listed on the labels. When mixing and applying pesticides, follow all label precautions to protect yourself and others around you. It is a violation of the law to disregard label directions. If pesticides are spilled on skin or clothing, remove clothing and wash skin thoroughly. Store pesticides in their original containers and keep them out of the reach of children, pets, and livestock.
YOU ARE REQUIRED BY LAW TO FOLLOW THE LABEL. It is a legal document. Always read the label before using any pesticide. You, the grower, are responsible for safe pesticide use. Trade (brand) names are provided for your reference only. No discrimination is intended, and other pesticides with the same active ingredient may be suitable. No endorsement is implied.
Articles from the Tree Fruit website may only be republished with prior author permission © Washington State University. Reprint articles with permission must include: Originally published by Washington State Tree Fruit Extension Fruit Matters at treefruit.wsu.edu and a link to the original article.



















