Soil Biota in Orchards
Written by: Tianna Dupont, WSU Tree Fruit Extension. posted: October 2018. Updated July 2019. FS315E – Download PDF
Introduction
The soil is alive. In just one acre of agricultural soil there can be 5,000 pounds of bacteria and fungi, 800 pounds of arthropods, 300 pounds of protozoa, and 100 pounds of nematodes (Kounang and Pimentel 1998). These organisms provide many ecosystem services that are essential for the healthy growth of your trees. For example, soil biota suppress pests; mineralize, scavenge, and cycle nutrients; and decompose plant and animal material, all ecosystem services which benefit orchard productivity.
Orchard floor management can increase the abundance and activity of soil biological communities. Organic matter additions from cover crops, crop residues, compost, and other material feed the soil food web. Practices such as mulching tree rows with wood chips or mown-and-blown cover crops as well as compost applications have been seen to increase soil biota in orchards and the ecosystem services they provide.
Bacteria
Bacteria are the smallest but most numerous organisms in soil. Bacteria are tiny, one-celled organisms, generally 4/100,000th of an inch wide (1 µm) and somewhat longer in length. What bacteria lack in size, they make up in numbers. A teaspoon of productive soil generally contains between 100 million and one billion bacteria. That is as much mass as two cows per acre.
Bacteria fall into four main groups. Most are decomposers that consume things like root exudates and litter and help to break them down. As they consume nutrients moving into soil they hold them in their bodies and help “immobilize” nutrients like nitrogen so that they do not go out of the root zone. Another group of bacteria are called mutualists because they work with plants or other organisms in partnerships, such as those in plant roots that fix nitrogen. Other mutualists are plant pathogens. The last group are called lithotrophs, and they use inorganic materials (such as sulfur and nitrogen) for their energy source, instead of carbon. These play an important role in nutrient cycling.
Examples of ecosystem services provided by bacteria:
Microbial Antagonism: Some bacteria excrete antibiotics. High populations of these bacteria in the root zone can help reduce infection by pathogens. Antibiotics are common among Streptomyces and are also common among numerous strains of Micromonespora and Nocardia.
Fungi
Fungi are composed of microscopic cells that usually grow as threads or strands, called hyphae, which push their way between soil particles, rocks, and roots. A single hypha can stretch from a few inches to a few miles.
Examples of ecosystem services provided by fungi:
Nutrient scavenging: One important group of fungi are called mycorrhizae. In particular vesicular arbuscular mycorrhizae (a type of mycorrhizae where the fungi penetrate the cortical cells of the roots) are important. They form a relationship with plant roots where the plant gives the fungi food (carbon), and the mycorrhizae scavenge for phosphorus, nitrogen, micronutrients, and water. Hyphae are much thinner than roots and can reach areas the roots cannot. Mycorrhizae in orchards are not well studied but may be important in phosphorus-limited soils (note: phosphorus limitation is not common in Washington orchards). In one study, Gąstoł et al. (2016) found apple plants with arbuscular mycorrhizal associations had higher nitrogen, potassium, phosphorus, and boron in shoots and nitrogen, sulfur, copper, and iron in roots than plants without.
Pest suppression: Orchard soils higher in Trichoderma fungi (as well as Streptomyces and Pseudomonas bacteria) have been found to be more suppressive to the pathogens involved in replant disease than soils with lower levels of beneficial fungi (Weerakoon et al. 2012). Abundant vesicular arbuscular mycorrhizae have also been found to successfully increase resistance to soil borne disease, resulting in more shoot growth in replant situations (Taube-Baab and Baltrushat 1993).
Decomposition: Fungi are particularly good at breaking down complex substrates, such as wood, because they exude compounds, like ligninase, that chemically break down cell walls.
Protozoa
Protozoa are single-celled organisms. They include flagellates, which move through the soil solution with the help of a whip-like structure called a flagella, cilates, which use hair-like structures called cilia, and amoebae. They are predominant in forest soils, but also abundant in agricultural soils.
Examples of ecosystem services provided by protozoa:
Nutrient cycling: Protozoa are important nutrient cyclers, providing nitrogen in plant-available forms as they consume bacteria in the soil (Griffith 1994). Protozoa have also been shown to enhance plant growth through mechanisms not related to nutrients, such as stimulation of plant hormones (Birkhoffer et al. 2008). In one study of orchard soils in British Columbia, Canada, protozoa numbered between 72 and 270 thousand per gram of soil (Forge et al. 2003). Numbers were higher in treatments with large carbon inputs.
Nematodes
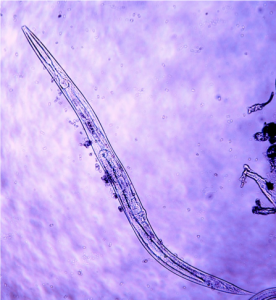
Nematodes are non-segmented, cylindrical round worms. Nematodes are one of the most numerous organisms in the world, often exceeding millions per square meter of sol. While nematodes inhabit a wide range of environments, including marine and freshwater, in agricultural systems we are most interested in the microscopic nematodes which inhabit the water films in soil. Fungal feeding and bacterial feeding, predatory, and omnivorous nematodes provide a number of services including nitrogen mineralization and plant pathogen suppression. In an on-going study in Washington State, orchard soils average 800 million nematodes per acre in the top six inches. (Average of 79 nematodes per 100 grams of dry soil from apple orchards, including bacterial feeding, fungal feeding, plant parasitic, predacious, and omnivorous nematodes. Assuming 2,000,000 pounds [1,000 tons] of soil in the top six inches of an acre) (DuPont and Kalcsits, unpublished data).
Example of ecosystem services provided by nematodes:
Nitrogen mineralization: Bacterial and fungal feeding nematodes are important because, like protozoa, when they eat bacteria and fungi they excrete nitrogen in a plant-available form (ammonia) (Figure 1). Predators and omnivores also release nitrogen as they consume other organisms. In grasslands, nematodes can mineralize as much as 8-22% of available nitrogen (Elliott et al. 1988), and in one study, nematodes mineralized 28 lbs/acre/year (Culman et al. 2010). One orchard study found nitrogen fluctuations due to mineralization of 97 to 617 mg N/kg of soil per year by bacterial/fungal-feeding nematodes and protozoa (Forge et al. 2003). One way to measure the nutrient cycling potential of soil is by looking at the proportion of bacterial- and fungal-feeding nematodes compared to other nematodes in the soil. This measurement is called the Enrichment Index (EI).
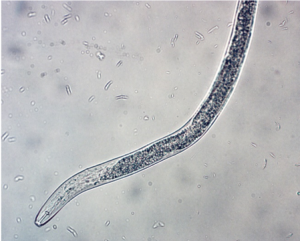
Plant parasitism: In orchards, lesion nematode (Pratylenchus) (Figure 2), dagger nematode (Xiphenema), and ring nematode (Criconema) can cause economic damage. Lesion nematode is known to be part of the replant complex. It can directly impact root growth and nutrient uptake by disrupting cells as it feeds as well as provide an entry point for other fungi and bacteria to infect tree roots. Dagger nematode can vector Tobacco ringspot virus causing apple union necrosis and apple decline as well as Cherry rasp leaf virus, which causes rasp leaf disease, strains of Tomato ringspot virus, which cause yellow bud mosaic, cherry mottle leaf, and Prunus stem pitting diseases.
Pest Suppression: Nematodes can also indicate the structure and complexity of soil food webs. Soils with a larger proportion of omnivorous and predatory nematodes tend to have larger amounts of other beneficial organisms as well, and they provide pest suppression and soil food web stability. In one study of apple orchard soild in British Columbia, certain mulch treatments led to larger proportions of the omnivorous/predatory nematodes and also to significantly lower levels of root lesion nematode (Forge et al. 2003). Some nematodes will attack insects (e.g., Steinernema) and may be useful in pest management, where a part of the life cycle is spent maturing in the soil, as with overwintering codling moth larvae (Lacey et al. 2006).

Microarthropods
Soil microarthropods are abundant small invertebrates that live in the soil and litter layer. Typical microarthropods include mites, springtails, pseudoscorpions, and insect larvae. These microarthropods can be important in controlling the rate of litter decomposition and altering nutrient cycling. In an on-going study, mites and collembolans counted in the top four inches of orchard soil ranged from zero to 25,000 per meter square (DuPont and Kalcsits, n.d.).
Collembola
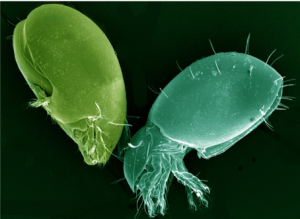
Collembola or “springtails” live in the litter and soil pores in the upper four inches of the soil (Figure 3). They feed mainly on fungi, bacteria, and algae growing on decomposing plant litter. Only a few millimeters in length, they are able to escape danger by “springing” high into the air with a forked tail-like furcula.
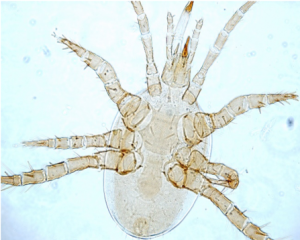
Fungal Feeding and Predator Mites
Soil mites such as Oribatids (Figure 4) eat fungi, algae, and dead plant material. These mites are extremely important. They break down dead leaves and other material, returning nutrients to the soil. Predator mites such as Mesostigmata (Figure 5), suppress pests and mineralize nutrients as they eat their prey.
Examples of ecosystem services provided by microarthropods:
N mineralization: Mesofauna feeding on microbes excrete nitrogen-rich waste contributing up to 30% of mineralized N (Deruiter et al. 1993; Griffiths 1994), and increasing plant uptake up to 50% (Laakso et al. 2000).
Pest suppression: Predation by Mesostigmata and other predacious mites can help keep pests in check.
Macroarthropods
Ground beetles (Carabidae) (Figure 6) and Rove beetles (Staphylinidae) are highly mobile generalist predators found in the leaf litter. Carabids in orchards can be highly diverse with 30 to 60 species. (Cross et al. 2015).
Examples of ecosystem services provided by macroarthropods:
Pest suppression: Codling moth, oriental fruit moth, apple maggot fly, apple leaf midge, plum curculio, wooly apply aphid, cherry fruit fly, and others spend part of their life cycle in the soil or leaf litter where they can be attacked by beetles and spiders. A number of studies have shown significant predation of codling moth by carabid beetles (de Roince et al. 2012; Hagley and Allen 1988). For example, in a small study, 34–81% of fourth- and fifth-instar codling moth larvae were attacked by beetles and other predators on the ground of an apple orchard (Mathews et al. 2004). Spiders, such as wolf spiders are also active predators.

Management
In order to increase the ecosystem services provided by soil biota in orchards, we can manage the orchard floor: providing food resources and limiting disturbance.
Feed the soil: Adding organic matter to soil is essential to promote active abundant soil biological communities. Cover crops, leaves, compost, prunings, manure, wood chips—all these organic materials that we add to soil are the food for these organisms (Caigelli 2012; Janvier 2007). Which type of organic material we add to soil changes which type of organisms will have the largest numbers. For example, adding material very high in carbon will encourage fungi that excrete enzymes such as chitinase, which can break down tough-to-digest material.
Manage disturbances: Some soil perturbations are a normal part of soil processes or are a necessary part of agriculture and other land uses. However, some disturbances significantly impact soil biology and can be minimized to reduce their negative effects. Tillage is a particularly important disturbance affecting soil biology. For example, surface tillage may destroy tree feeder roots and beneficial fungal hyphae.
Compaction: Ideally, soils are approximately 50 to 60% pore space comprising a variety of pore sizes and lengths. The size and continuity of pores control whether larger microbes, such as protozoa, can prey upon bacteria and fungi. Compaction reduces the diversity of pore sizes and the amount of space and pathways available for larger organisms (Figure 2) to move through the soil. This favors bacteria and small predators over fungi and the larger predators.
Arthropods are severely affected by compaction. Among nematodes, the predatory species are most sensitive to compaction, followed by fungal feeders, then bacterial feeders. Root-feeding nematodes are least sensitive to compaction—perhaps because they do not need to move through soil in search of food. Compaction changes the movement of air and water through soil, can cause a shift from aerobic to more anaerobic organisms, and may increase losses of nitrogen to the atmosphere (denitrification). Rooting depth may be limited in highly compacted soils. Compaction restricts the depth of the rhizosphere environment that supports microbes.
Pesticides: There is some literature showing negative impacts of pesticides on soil biota, such as to microarthropods (Doles et al. 2001; Brown and Adler 1989; Brown and Puterka 1997). However, this relationship can be complicated with some pesticides, such as herbicides, providing a flush of microbial growth in response to readily available resources. Declines in soil biota in orchard soils with continual herbicide use (Yao et al. 2005) is more likely due to the lack of carbon inputs into the soil than toxic effects of the herbicides themselves.
Other: Orchard floor management can affect soil biology. Keeping the soil covered and protecting living roots generally encourages soil biology and biota both by improving habitat (lessening environmental extremes) and increasing food source (root exudates, dead roots, plant litter). In contrast, plastic mulch negatively affected carabid beetle numbers (Mathews et al. 2004) and soil microbes (Forge et al. 2003) in two orchard floor studies.
Indicators
There are many indicators of soil biological function. We will describe a few here.
Soil Protein Index is an indicator of the fraction of soil organic matter that is present as proteins. This organically bound nitrogen can be mineralized by soil biota and become available for plant uptake. It is an indicator of the soil’s ability to store N and is associated with microbial activity as well as aggregate stability.
Soil respiration is a measure of the metabolic activity of the soil microbial community. There are a number of protocols for measuring soil respiration. Cornell quantifies soil respiration by measuring CO2 released from re-wetted soil held in an airtight jar for four days. However, respiration can change rapidly (e.g., from dry to wet soil, with a management intervention like compost) and thus it may be difficult to interpret the results with confidence.
Active carbon is the portion of the soil organic carbon that is readily available as a food source for microbes. It is generally directly related to the size and activity of the microbial community, yet its level does not vary as quickly as the microbial biomass. It is measured by a reaction with permanganate.
Nematode analysis is done by washing nematodes from soil and using Baerman funnels and centrifugation to concentrate nematodes in water where they are counted under the microscope.
References
Birkhofer, K., T.M. Bezemar, J.Bloem, M. Bonkowski, S. Christensen, D. Dubois, F. Ekelund. 2008. Long-term organic farming fosters below and above ground biota: Implications for soil quality, biological control and productivity. Soil Biology & Biochemistry 40(9):2297-2308.
Brown, M. W., and C.R.L. Adler. 1989. Community structure of phytophagous arthropods on apple. Environmental Entomology 18 (4):600-607.
Brown, M. W., and B. J. Puterka. 1997. Orchard management effects on the arthropod community on peach with comparison to apple. (vol 32, pg 165.). Journal of Entomological Science 32(3):373-373, pg 165.
Caigelli, M. A. 2012. Managing soil biodiversity and ecosystem services. In Soil Ecology & Ecosystem Services. edited by D. H. Wahl. pg 337–356. London: Oxford University Press.
Cross, J., M. Fountain, V. Marko, and C. Nagy. 2015. Arthropod ecosystem services in apple orchards and their economic benefits. Ecological Entomology 40:82-96.
Culman, S. W., S. T. DuPont, J. D. Glover, D. H. Buckley, G. W. Fick, H. Ferris, and T. E. Crews. 2010. Long-term impacts of high-input annual cropping and unfertilized perennial grass production on soil properties and belowground food webs in Kansas, USA. Agriculture Ecosystems & Environment 137(1-2):13-24.
de Roince, C. B., C. Lavigne, J. M. Ricard, P. Franck, J. C. Bouvier, A. Garcin, and W. O. C. Symondson. 2012. Predation by generalist predators on the codling moth versus a closely-related emerging pest the oriental fruit moth: a molecular analysis. Agricultural and Forest Entomology 14 (3):260-269.
Deruiter, P. C., J. A. Vanveen, J. C. Moore, L. Brussaard, and H. W. Hunt. 1993. Calculation of Nitrogen Mineralization in Soil Food Webs. Plant and Soil 157(2):263-273.
Doles, J. L., R. J. Zimmerman, and J. C. Moore. 2001. Soil microarthropod community structure and dynamics in organic and conventionally managed apple orchards in Western Colorado, USA. Applied Soil Ecology 18(1):83-96.
DuPont, S.T., and L. Kalcsits. n.d. Project: How do We Measure and Manage Soil Health for Productive Orchards? Washington Stte University. Wenatchee, WA.
Elliott, E. T., H. W. Hunt, and D.E. Walter. 1988. Detrital foodweb interactions in North-American grassland ecosystems. Agriculture Ecosystems & Environment 24:41-56.
Forge, T.A., E. Hogue, G. Neilsen, and D. Neilsen. 2003. Effects of organic mulches on soil microfauna in the root zone of apple: implications for nutrient fluxes and functional diversity of the soil food web. Applied Soil Ecology 22:39-54.
Gąstoł, M., I. Domagała-Świątkiewicz, and M. Bijak. 2016. The effect of mycorrhizal inoculation and phosphorus application on the growth and mineral nutrient status of apple seedlings. Journal of Plant Nutrition 39(2):288-299.
Griffiths, B. S. 1994. Microbial feeding nematodes and protozoa in soil. Their effects on microbial activity and nitrogen mineralization in decomposition hot spots and the rhizosphere. Plant and Soil 164(1):25-33.
Hagley, E. A. C., and W. R. Allen. 1988. Ground beetles (Coleoptera, Carabidae) as predators of the codling moth, Cydia pomonella (Lepidoptera, Tortricidae). Canadian Entomologist 120(10):917-925.
Janvier, C. 2007. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biology Biochemistry 39:1-23.
Kounang, N, D. Pimentel, and N. Kounang. 1998. Ecology of Soil Erosion in Ecosystems. Ecosystems 1(5):416-426.
Laakso, J., H. Setala, and A. Palojarvi. 2000. Influence of decomposer food web structure and nitrogen availability on plant growth. Plant and Soil 225(1-2):153-165.
Lacey, L. A., D. Granatstein, S. P. Arthurs, H. Headrick, and R. Fritts Jr. 2006. Use of entomopathogenic nematodes (Steinernematidae) in conjunction with mulches for control of overwintering codling moth (Lepidoptera: Tortricidae). Journal of Entomological Science 41:107-119.
Mathews, C. R., D. G. Bottrell, and M. W. Brown. 2004. Habitat manipulation of the apple orchard floor to increase ground-dwelling predators and predation of Cydia pomonella (L.) (Lepidoptera: Tortricidae). Biological Control 30(2):265-273.
Taube-Baab, H., and H. Baltrushat. 1993. Effect of vesicular-arbuscular mycorrhizal fungi on the growth of young apple trees in apple replant disease soil. Journal of Plant Diseases and Protection 100(5):474-481.
Weerakoon, D. M. N., C. L. Reardon, T. C. Paulitz, A. D. Izzo, and M. Mazzola. 2012. Long-term suppression of Pythium abappressorium induced by Brassica juncea seed meal amendment is biologically mediated. Soil Biology & Biochemistry 51:44-52.
Yao, S., I.A. Merwin, G.W. Bird, G.S. Abawi, and J.E. Thies. 2005. Orchard floor management practices that maintain vegetative or biomass groundcover stimulate soil microbial activity and alter soil microbial community composition. Plant and Soil 271(1–2):377–389.
