Written by Louis Nottingham and Katlyn Catron, August 9, 2021
Introduction
Data showing efficacy of insecticides against leafhopper vectors of x-disease is still relatively sparse. To address this, we performed two lab bioassays to screen insecticide products against Colladonus montanus reductus, the most abundant leafhopper vector of x-disease found in Washington cherries. We evaluated insecticides in both direct-spray and residue decline experiments to assess the immediate and longer-term efficacy of these products on the leafhoppers.
Contact Spray Lab Bioassay – 2021
Methods
Leafhoppers (C. reductus) were collected from weedy row middles in an organic commercial apple orchard bordering a conventional cherry block and returned to the lab for processing. They were sorted into bioassay arenas composed of an 8 oz plastic deli cup with a layer of moist soil on the bottom, an excised cherry leaf, and a lid with a plastic mesh cutout to allow for application of treatments and air circulation (Fig. 1). Approximately six leafhoppers were placed into each assay arena, then treatments (Table 1) were applied through the mesh cutout on the deli cup lid using a hand-pump aluminum spray bottle. Five replicates per treatment were completed. Assay arenas were moved to a greenhouse for 24 hours before mortality was evaluated.
Results and Discussion
All products provided significant control compared with the check, and were statistically similar (Table 2). Numerically, the group 4A (neonicotinoid) insecticides, Admire Pro and Actara, provided the highest efficacy at or near 100% mortality. The group 4C insecticide, Transform, also provided high efficacy at 90% (± 3.7%). Magister, a 21A METI, was on the lower range for these products, at 80% mortality. This was the first evaluation of Magister against leafhoppers, so additional testing is needed before recommendations are made.
Insecticide Residue Bioassay
Methods
To evaluate the longer-term efficacy of several insecticides, potted cherry trees were treated with insecticide products (Table 2) using hand-pump aluminum spray bottles. Trees remained exposed to outdoor ambient conditions, including sunlight and occasional rain, for the duration of the experiment. Treated cherry leaves were excised at predetermined intervals, after 0 h (fresh residues), 72 h (3 days), 168 h (7 days), and 336 h (14 days). After excision, leaves were placed in arenas as described in the Contact Spray Lab Bioassay above (Fig. 1), and approximately six C. reductus adults were exposed to the aged residues per replicate (3-5 replicates per treatment per time interval). After 24 hours, mortality was evaluated.
Results and Discussion
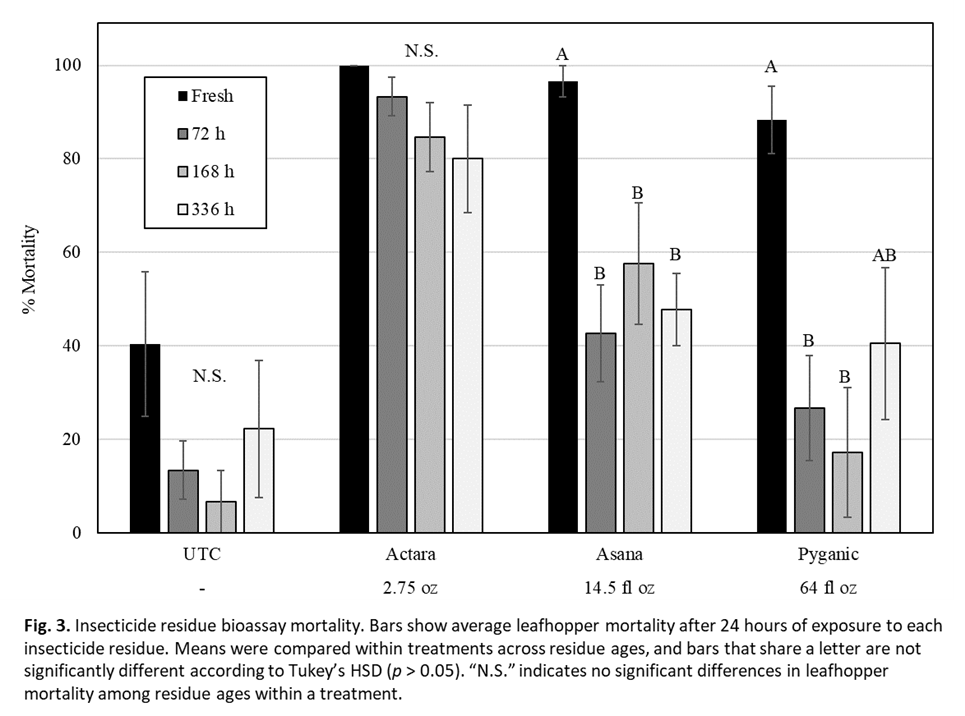
C. reductus mortality after exposure to fresh insecticide residues ranged from 88.3% in the Pyganic (pyrethrins) treatment to 100% in the Actara (thiamethoxam) treatment (Fig. 3). Mortality in leafhoppers exposed to Actara remained higher than 80% even after two weeks (336 h) of residue aging outdoors. In the Asana (esfenvalerate) and Pyganic treatments, leafhopper mortality dropped considerably after 72 h of residue aging, with neither insecticide causing greater than 60% mortality when aged. Pyrethroids are notoriously unstable when exposed to UV light, which explains the reduced efficacy of Pyganic after treated leaves were exposed to outdoor conditions for 72+ hours. Asana provided slightly better control after 72+ hours of residue aging, which may be due to its oil-based formulation specifically designed to withstand UV rays and rain wash-off. Regardless, Actara provided the highest levels of control for the longest periods of time in this bioassay.
Conclusions
The results from these trials will inform future insecticide work with leafhopper vectors of X-disease in Washington. In the next year, additional products will be screened in both direct-spray and aged residue bioassays to help form a more complete picture of control methods available to and effective for growers. Furthermore, other economically relevant species of leafhoppers will be incorporated into similar trials to assess their susceptibility to these insecticides.
Acknowledgements:
Thanks to our funding from the Washington Tree Fruit Research Commission (Cherry), Oregon Sweet Cherry Commission, Corteva and Gowan for support of this research.
Contact
 Katlyn Catron
Katlyn Catron
Postdoctoral Research Associate
WSU TFREC
katlyn.catron@wsu.edu

Louis Nottingham
Research Assistant Professor
WSU TFREC
louis.nottingham@wsu.edu
Use pesticides with care. Apply them only to plants, animals, or sites listed on the labels. When mixing and applying pesticides, follow all label precautions to protect yourself and others around you. It is a violation of the law to disregard label directions. If pesticides are spilled on skin or clothing, remove clothing and wash skin thoroughly. Store pesticides in their original containers and keep them out of the reach of children, pets, and livestock.
YOU ARE REQUIRED BY LAW TO FOLLOW THE LABEL. It is a legal document. Always read the label before using any pesticide. You, the grower, are responsible for safe pesticide use. Trade (brand) names are provided for your reference only. No discrimination is intended, and other pesticides with the same active ingredient may be suitable. No endorsement is implied.