Written by Alexis Hamilton, Research Assistant, Washington State University Irrigated Agriculture Research and Extension Center, Prosser, WA;
Faith Critzer, Associate Professor, University of Georgia, Athens, GA
Summary
Gray mold is one of the four most prevalent fungal species observed to contribute to postharvest fungal losses of fresh apples in Washington State. The disease is caused by Botrytis cinerea, a fungal pathogen that works to decay apple fruit as part of its infection strategy. This can create an environment that is suitable for Listeria monocytogenes growth and survival. Since Listeria is known to grow at storage temperatures and its presence indicates adulteration in the apple product, it is important to understand how these two organisms interact to impact food safety risks during storage. Fresh ‘Gala’ apples were harvested in 2019, inoculated with Listeria and Botrytis species, and placed in storage for up to 11 months. Listeria persisted best on wounded apples inoculated with B. cinerea. The apple industry can minimize the risks associated with Botrytis infection by implementing good harvest and postharvest handling practices, optimizing fungicide programs to effectively target Botrytis species, minimizing opportunities for fungal inoculum spreading and overwintering in the orchard, and facilitating the removal of physically wounded or visibly decayed apples in or before entry into the packinghouse environment.
Introduction
Recent estimates of postharvest losses in Washington State that were due to fungal decay have reached levels as high as 80%, with gray mold emerging as one of the four most prevalent causes. Gray mold is caused by Botrytis cinerea, a necrotic pathogen known to overwinter in dead and dying leaf and host tissue on the orchard floor. Listeria monocytogenes is a foodborne pathogen known to thrive in decaying environments and able to grow at temperatures used in long-term apple storage. The industry currently carries a zero tolerance for this organism in the crop or on packinghouse facility surfaces, so indicator organisms like Listeria innocua are commonly used as a means of monitoring risk in production environments. Since it is difficult to monitor and control for L. monocytogenes directly, identifying a link between foodborne pathogen growth patterns and apple fungal pathogens may provide an additional means of preventing large-scale contamination events across the industry.
The objective of this project was to observe population changes of L. innocua as a common surrogate for L. monocytogenes on wounded and unwounded apples inoculated with and without Botrytis cinerea during industry-relevant long-term controlled atmosphere cold storage conditions. This study sought to identify if the presence of a fungal pathogen on fresh apples impacted growth patterns of a food safety-relevant microorganism.
Methods
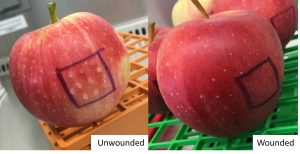
‘Gala’ apples were harvested from one of Washington State University’s experimental orchards in the Columbia Basin, treated with pyrimethanil, and screened for surface defects (stem punctures, bird pecks, etc.). Market quality apples were wounded on one side and separated into two treatments: a L. innocua-only control (n = 90) and L. innocua/B. cinerea co-inoculation (n = 90). Apples were inoculated with L. innocua on wounded and unwounded sides and allowed to dry in a biosafety cabinet (Figure 1). Bins were treated with 1-methylcyclopropene and stored at 2kPa oxygen, 1kPa carbon dioxide, and 1°C to simulate conditions used by the industry for long-term controlled atmosphere cold storage. After 6, 9, and 11 months of storage, bins were removed, and samples were evaluated for the presence of L. innocua based on polymerase chain reaction (PCR) analysis. These data were analyzed by timepoint, treatment, and wound status using nominal logistic regression. The relationships between these variables and L. innocua detection were described using odds ratios.
Fig 1. Example of inoculated ‘Gala’ apples on unwounded (left) and wounded (right) sample surfaces.
Results
L. innocua was detected on ‘Gala’ apples through eleven months of storage (Table 1). The odds of detection significantly decreased the longer apples were storage. This decline occurred faster on unwounded apple samples, where the odds of Listeria detection were 4.12 times greater on wounded compared to unwounded apples. Persistence was greater on both unwounded and wounded samples for apples that were co-inoculated with B. cinerea than those with L. innocua only; although, persistence was almost ten times greater on wounded samples.
Table 1. Percent of samples that contained detectable Listeria innocua.
| Months in Storage (Days) | Percent Detection (Positive Samples/Total Samples) | |||
| L. innocua only | L. innocua + B. cinerea | |||
| Unwounded | Wounded | Unwounded | Wounded | |
| 6 (183) | 100.0 (30/30) | 96.7 (29/30) | 76.7 (23/30) | 100.0 (30/30) |
| 9 (272) | 60.0 (18/30) | 86.7 (26/30) | 60.0 (18/30) | 90.0 (27/30) |
| 11 (334) | 3.3 (1/30) | 53.3 (16/30) | 6.9 (2/29)a | 82.8 (24/29) |
| aWhen fungal growth from one side of the apple crossed over to the sample area on the opposite side, those apples were considered compromised and discarded. | ||||
Discussion
L. innocua was able to survive on fresh apple surfaces through the complete duration of the storage time, suggesting that the food safety risks present prior to storage can be carried into the packinghouse environment at any time. However, L. innocua was detected on fewer apples over time. Additionally, the greater detection on wounded apples emphasizes the importance of implementing good harvest and postharvest handling practices to cull damaged apples before they enter storage as an important strategy. Listeria persisted better on apples that were also inoculated with B. cinerea, which indicated that Botrytis-infected environment enhanced Listeria persistence. This suggests that food safety risks in the storage environment can differ based on fungal presence and change over time.
When possible, apples with visible surface defects like bird pecks, stem punctures, limb rub, etc. should be removed from the harvest bin prior to storage or as soon as possible prior to packing. Additionally, to mitigate the protective effect that Botrytis cinerea provides for L. innocua on fresh apples in storage, industry should prioritize three additional practices: (1) optimize fungicide programs to effectively target fungal pathogens like B. cinerea, (2) minimize opportunities for fungal inoculum spreading in the orchard environment through removing decay fruit and organic debris from orchard floors, and (3) facilitate the removal of physically wounded or visibly decayed apples as early as possible during packing.
Additional Information
The data presented in this article is part of a larger research study that has been accepted for publication in the Journal of Food Protection. Please reach out to the authors for article information.
Click here for additional content about gray mold management.
Fruit Matters articles may only be republished with prior author permission © Washington State University. Reprint articles with permission must include: Originally published by Washington State Tree Fruit Extension Fruit Matters at treefruit.wsu.edu and a link to the original article.