by Jay F. Brunner, Stanley C. Hoyt, and Elizabeth H. Beers, originally published 1993
What is IPM?
Integrated pest management (IPM) is a philosophy of pest control founded on the principles of ecology. In practice, it involves using several control tactics based on a knowledge of the crop, pests and associated natural enemies to avoid crop loss and minimize harmful effects on the environment. Implementing IPM requires an understanding not only of insect and mite biology and ecology but also of the entire orchard system. This includes the plants and animals that comprise the orchard community, as well as consideration of contributions from the surrounding habitat. The orchard system also takes into account financial, physical and human aspects of orchard operations.
IPM requires a more tolerant approach to pest control than traditional insecticide-based programs. Eliminating all insects and mites from the orchard is not the objective of IPM. Natural enemies are to be conserved as much as possible and some damage, especially to foliage, is tolerated. For example, pests that attack the foliage can usually be allowed to build to levels higher than those that attack the fruit.
There are both positive and negative impacts associated with the reduced insecticide use that usually accompanies the adoption of an IPM approach. Benefits of IPM include greater survival of natural enemies, slower development of resistance, less pest resurgence, fewer outbreaks of secondary pests, less negative impact on the environment, and greater worker safety. On the negative side, potential pests that are coincidentally controlled by insecticides used to control key pests may be released from all but natural controls. Natural controls will be effective for some. For others, however, the release from insecticidal control will result in population levels that are sometimes damaging. The transition to more intensive IPM programs in orchards will require knowledge and patience-knowledge of pest and natural enemy biology and patience to allow natural enemy build-up. Selective controls will have to be used for pests that are not maintained at acceptable levels by natural controls.
An IPM program involves:
- Identifying pests, which requires knowledge of their biology and the damage they inflict.
- Identifying the natural enemies of pests.
- Understanding the biological and environmental factors that affect the abundance and distribution of pests and natural enemies.
- Monitoring both pests and natural enemies to determine potential for damage and biological control.
- Tolerating higher levels of pests, particularly foliage feeders.
- Using a treatment threshold to decide when control is needed.
- Knowing the efficacy of available control tactics, as well as their potential impact on non-target pests and natural enemies.
- Building flexibility into the control program to allow for variations from block to block or year to year.
- Follow-up to see how well control measures work and if further action is needed.
History
The advent of synthetic insecticides after World War II launched a new era of pest control. The number of registered pesticides rose from about 30 in 1936 to more than 900 in 1972. The new chemicals were effective, easy to use and inexpensive. For several years it appeared that broad-spectrum pesticides could eliminate most pest problems. Sprays were often used on a routine, preventive basis, providing protection for the crop whether the insect was there in damaging numbers or not.
Soon, however, insects began to develop resistance to insecticides, and new problems arose because natural enemies were eliminated. Some insects that previous to broad-spectrum insecticides had been kept in check by their natural enemies now reached pest status. In the absence of natural enemies, growers often applied more toxic products in an effort to control these secondary pests, as well as resurging populations of the insecticide-resistant target pest. Orchardists became trapped in a cycle of using more insecticides to cure one pest problem, which resulted in the worsening of another pest problem. The cost of control increased while the degree of control often declined and the harmful effect on the environment escalated.
The inability to control mites during the 1950s and 1960s was a major impetus in the development of integrated mite control in apple orchards. Mites were quickly developing resistance to miticides. Scientists found that predatory mites had developed resistance to some organophosphate insecticides and, if these were used at selective rates and properly timed, biological control of mites could be integrated with chemical control of other insects. It meant tolerating some mites in the orchard until predators gained control and at first was a difficult concept for growers to accept. But integrated mite control became so successful that many orchardists in the Pacific Northwest have not had to apply chemicals to control mites since the 1960s.
Although integrated mite management was widely adopted in the early 1970s, growers have continued to rely almost exclusively on insecticides for control of other pests. Insecticides have been effective and in the absence of any crisis, as was experienced with mites, IPM was a less attractive alternative. Recently, however, the pace toward widespread implementation of IPM for the entire complement of orchard pests has been quickening. A number of factors are responsible for the renewed interest in IPM. Those most evident are:
- A decline in the number of insecticides available because of the EPA’s re-registration of old pesticides
- A lack of new registrations because of development costs and regulatory requirements
- Reduction in the effectiveness of registered products because of pest resistance.
The public’s concern over insecticide residues on food, contamination of the environment, and exposure of farm workers to insecticide residues during thinning, tree training, and harvesting operations are issues of increasing importance. IPM addresses each of these concerns. Moreover, IPM has the capacity to evolve to accommodate new pests and control techniques.
Integrating Management Tactics
A successful IPM program incorporates a variety of compatible tactics such as biological control, cultural control, judicious use of insecticides, and autocidal techniques such as mating disruption. It does not preclude the use of insecticides but attempts to use them as a last line of defense against pests, not as the first option for control. IPM recognizes that insecticides are one of many tools available for managing pests and the more tools that are included in a management program, the stronger it will be.
For example, where mating disruption is used to control codling moth, supplemental treatment with insecticides may be needed to reduce pest pressure on orchard borders to prevent damage resulting from mated moths immigrating from adjacent sources.
Conflicts often arise when attempting to integrate insecticidal and biological controls. However, these problems can be reduced by using insecticides that are selective, that is, those that control the pest but are less toxic to natural enemies. Applying insecticides only where needed and timing applications when the pest is most vulnerable will maximize benefits of the chemical control while reducing the impact on natural enemies. Delaying treatments as long as possible to allow predator and parasitoid populations to build up or using chemical controls during the dormant period before natural enemies are active are strategies that will help to encourage biological controls.
In an IPM program, pests are not treated in isolation. It is important to consider their relationships with other insects and with their environment. The distribution and abundance of pests are influenced by their natural enemies, weather, orchard practices including pruning, fertilization and cover crop management, and by habitats surrounding the orchard that may provide alternate hosts for pests and their natural enemies. Understanding that these factors influence pest populations is a key to successful pest management.
Similarly, decisions that affect the biological components of the orchard system are not made without considering the larger social system of which they are a part. The tree fruit industry is well aware of how pesticide laws and the concerns of consumers can dramatically impact production and marketing programs. IPM should provide the platform for developing a more harmonious relationship between the agricultural community and social and environmental action groups.
Components of an IPM program
Pest identification
The use of broad-spectrum insecticides reduces the need to know specifically what pest is causing what damage. Treatment decisions are often prevention-based, and several target pests are killed with the same treatment. As more selective controls are incorporated into a management program, it becomes increasingly important to know more about the target pest in order to achieve control. The effectiveness of selective controls often depends on very accurate timing of applications.
Damage in an orchard may not necessarily be due to the most abundant insect present at the time observations are made. An incorrect diagnosis can lead to unnecessary sprays. Many insects found in the orchard are not pests, but only incidental visitors, while others are beneficial, acting as biological controls for pests. Some pest and beneficial species look similar. For example, many stink bugs are potential pests, but a Brochymena species is predaceous and a natural enemy of soft-bodied pests in orchards.
Several kinds of information are helpful when identifying an insect in the field. Physical appearance (including color, size and shape) is usually of primary importance. However, since many pests restrict their feeding to only certain plant parts, knowing where they are most likely to be found helps narrow the possibilities when making field identifications. Similarly, many insects leave characteristic feeding damage that provides a clue to their identity. Descriptions and pictures of the most common insect and mite pests, their natural enemies, and typical damage caused by pests are presented in this manual.
Monitoring
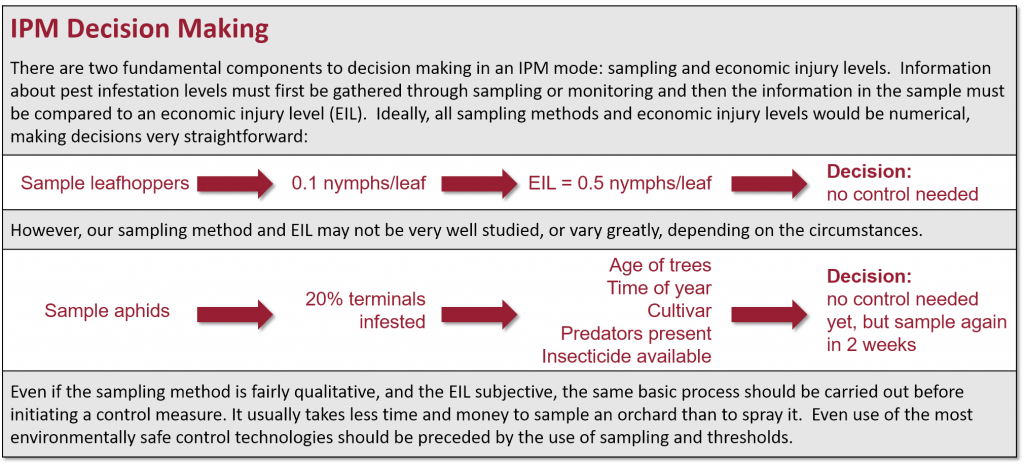
Monitoring is the most fundamental yet the most often neglected activity in an IPM program. Both the need for control and the effectiveness of any action taken are determined by monitoring pest and natural enemy populations. Since it is impossible to count all the insects, only a portion, a sample, is counted. Information obtained from the sample is used to make inferences about an insect’s density in the entire orchard. To decide if a control is required, pest density must be related to the potential damage and balanced against how likely it is that the pest’s natural enemies will be able to keep it below damaging levels. Even if treatment thresholds are unknown, sampling provides information on the insect’s stage of development, population densities and the ratio of pests to natural enemies, all of which form a sound basis for decision making. Management in the absence of sampling usually leads to an overuse of insecticides. It is important to know how a pest develops, i.e., its life history, because different life stages may be monitored and managed in different ways. For example, you would sample foliage to look for leafroller larvae, which feed on leaves and fruit, but use pheromone traps to monitor adults. Control decisions may be based on either of these monitoring methods at different times during the season (see Leafrollers, Direct Pests). Different sampling methods are used, depending on the type of pest and monitoring objective. Since all methods only estimate the number of individuals in the actual population, there is always variation from one sample to the next. This variation is kept within acceptable limits by guidelines that specify how, when, and where to sample. Detailed sampling guidelines for many orchard pests are presented in this manual. Some general methods of monitoring orchard pests and their natural enemies are outlined here.
Visual counts
Caterpillars, leafminers, leafhoppers, aphids, mites and many of their natural enemies can be sampled simply by counting them on leaves, shoots or fruit. A 10-power hand lens is an essential tool. It makes sampling smaller insects easier and is necessary when evaluating parasitism of leafminers.
Leaf brushing
Leaf brushing is the standard method for counting mites and can be used to estimate psylla densities. A machine is used to brush mites or immature pear psylla off leaves and onto a glass plate placed over a grid to make counting easier. See the appropriate pest section for the kind and number of leaves to be sampled and subsequently brushed.
Beating tray
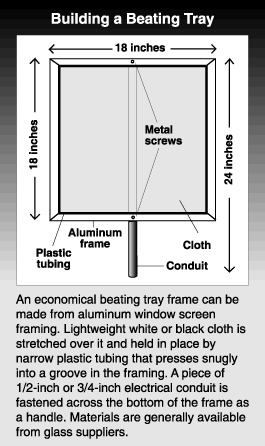 Insects such as adult pear psylla, plant bugs, older caterpillars, and predators of pear psylla and aphids can be monitored by jarring them from limbs onto a cloth tray. A white cloth provides a good background for counting most insects. However, light colored pests such as campylomma are more easily detected against a black cloth. The tray is held under an almost horizontal section of limb that is 3/4 inch to 1-1/2 inches in diameter with an average complement of branches and spurs. The limb is firmly tapped three times with a l-foot length of stiff rubber hose. Old spray hose works well. Insects jarred from the tree cling to the cloth and can easily be counted. Insects and debris are removed from the tray by turning it upright and tapping it lightly with the hose.
Insects such as adult pear psylla, plant bugs, older caterpillars, and predators of pear psylla and aphids can be monitored by jarring them from limbs onto a cloth tray. A white cloth provides a good background for counting most insects. However, light colored pests such as campylomma are more easily detected against a black cloth. The tray is held under an almost horizontal section of limb that is 3/4 inch to 1-1/2 inches in diameter with an average complement of branches and spurs. The limb is firmly tapped three times with a l-foot length of stiff rubber hose. Old spray hose works well. Insects jarred from the tree cling to the cloth and can easily be counted. Insects and debris are removed from the tray by turning it upright and tapping it lightly with the hose.
Beating tray counts should be taken at random throughout a block. The number of tray samples required to make a management decision varies and is discussed in the specific pest sections. When sampling for parasitic wasps or other minute insects, an aspirator can be used to collect the specimens for later identification.
Traps
Pheromone traps are a quick and convenient way to monitor many moth species, San Jose scale and campylomma. When used in conjunction with phenology models, they enable growers to apply controls at precise times in an insect’s life history rather than according to a calendar-based schedule. Pheromones are volatile chemical attractants produced by insects to communicate with their own species. Most pheromones used in traps are synthetic compounds that mimic those released by females to attract males for mating (see section on Mating Disruption and Degree-day Models). The main limitation of pheromone traps is that only the adult males can be monitored, and activity of males may not always be representative of female activity. Pheromone traps can be used to help:
- Monitor when adult flight begins (biofix), as well as peak flight and its duration
- Track seasonal development
- Determine when populations reach treatment thresholds
- Assess how well control programs are working
- Synchronize degree-day models with actual pest development
- Monitor the presence of exotic or introduced pests
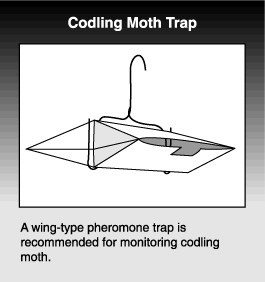 Traps of various designs and sizes are available commercially. Some are cylinder shaped, while others are referred to as wing or tent types. Most are made of cardboard and have an adhesive on the inside (Figure 29). A lure containing the pheromone is placed inside the trap. Pheromone is slowly released and insects are attracted to and caught in the trap’s adhesive surface. The number of insects that can be caught in a trap depends on the size of the sticky surface.
Traps of various designs and sizes are available commercially. Some are cylinder shaped, while others are referred to as wing or tent types. Most are made of cardboard and have an adhesive on the inside (Figure 29). A lure containing the pheromone is placed inside the trap. Pheromone is slowly released and insects are attracted to and caught in the trap’s adhesive surface. The number of insects that can be caught in a trap depends on the size of the sticky surface.
Choosing a trap system
The size and shape of the trap can make a difference in how efficiently it catches the target insect. The trap should be easy to assemble and efficient, and the lure should release pheromone at a constant rate. The trap system also should allow easy maintenance, such as removing trapped insects, replacing the adhesive catch surface or installing a new pheromone lure. A permanent trap with a replaceable adhesive liner can be used for several seasons and may be cheaper in the long run than a disposable trap used for one season. Permanent traps should be cleaned periodically to remove contaminants such as spray residues, which could make the traps repellent to the pest.
Placement
Place traps in the orchard before the pest starts to emerge. Hang them within the tree canopy as recommended for the pest being monitored. The number of traps needed depends on why they are being used. A few strategically placed traps are all that is needed to establish biofix for degree-day models. More traps are needed if the objective is to assess a pest’s distribution and density. To assess pest density, traps should be placed in a grid pattern. Usually the number of moths caught per trap will increase as the number of traps within an area decreases. This relationship should be taken into account when comparing data from different orchards using different trapping densities. It also can be useful to place traps on the border of the orchard to monitor movement of insects into the orchard. If the number of insects captured in border traps is higher than interior traps, it usually signals problems from an external source. When using the number of insects captured in pheromone traps as a treatment threshold, always use the trap density specified.
Maintenance
Traps should be checked at regular intervals, at least weekly. As the season progresses, the sticky surface inside the trap can become contaminated with moth scales, debris, dust or nontarget insects. Any deterioration of the trap’s adhesive surface will reduce its efficiency. Contaminated adhesive surfaces should be changed as needed. For example, the efficiency of the wing-type trap declines after an accumulated catch of about 30 moths. When trapping in blocks where pest densities are low, as is often the case with codling moth, the adhesive should be stirred after removing insects to maintain the efficiency of the trap.
Interpreting data
Though the pheromone trap is easy to use, it may not be as easy to interpret the results. Moth capture can be influenced by:
- Density of the male insect population
- Age of the male insects
- The effect of wind and slope on male movement
- Competition from calling females
- Trap design and size
- Condition of the pheromone lure
- Trap maintenance, placement and density
For these reasons, moth capture in a pheromone trap provides only a rough estimate of pest density. However, if pheromone traps are used properly throughout the season and from one year to the next, they can provide useful comparative data.
Other types of traps can be used to monitor insects, including sticky traps (with or without attractants), light traps and bait traps. Two kinds of sticky traps are used to monitor flies. They are a yellow panel baited with ammonium acetate and casein hydrolysate or ammonium carbonate, and a red sphere. The cherry fruit fly, a relative of the apple maggot, is usually monitored with a baited yellow panel. Pails containing an ammonia-, molasses- or terpinyl acetate-based liquid bait can be used to trap several species of moths. Moths are also attracted to light sources placed in the orchard. Little is known about the effectiveness of most bait or light traps so their use in estimating pest density has limited value.
Degree-day models
Degree-day models, when coupled with monitoring, enable you to determine the most effective time for treatment applications. Insect development is influenced by temperature, and treatments applied on a calendar basis can often be poorly timed due to fluctuations in weather from season to season. Many selective controls, such as insect growth regulators and microbial insecticides, must be applied at a precise time in the pest’s life cycle to be effective.
A degree-day model predicts insect development by accumulating heat units (degree-days) and associating these with critical events in its life cycle. Models are usually initiated at some easily monitored event (biofix), such as first capture of males in pheromone traps. Thereafter, daily temperatures are used to calculate degree-days specific to that insect. Degree-day tables for some key tree fruit pests are available on this web site. The primary benefit of degree-day models is their ability to predict critical events in the insect’s life history that would be difficult to observe directly.
Decision making
The presence of an insect in the orchard is not sufficient justification, in most cases, to initiate control measures. If an insect is a potential pest, the severity of the problem depends on the type of damage it causes and how much damage the grower is willing or able to accept. For example, indirect (foliage-feeding) pests such as western tentiform leafminer and white apple leafhopper can be tolerated in higher numbers than direct pests such as codling moth, which attack fruit. In addition, it may pay long-term dividends to accept a small amount of fruit injury from a pest such as pear psylla to allow natural enemy populations to increase, achieving biological control and thus minimizing the effects of pest control activities on the environment.
The economic injury level is defined as the pest density that causes damage equal in value to the cost of control. In other words, it is the lowest pest level at which control becomes economically feasible by weighing the cost of potential losses due to crop damage against the cost of control. The economic injury level will be different for different cultivars and will vary with tree vigor, crop load and the time of year. In addition to biological components, growers must also factor in the costs and profitability of their own orchard operations.
Economic injury levels for direct pests (where a damaged fruit is culled) are usually easier to establish than those for indirect pests. In reality, economic injury levels have been determined for very few fruit pests.
The treatment (or action) threshold is the density at which control measures must be applied to prevent pest densities from exceeding the economic injury level. The treatment threshold is lower than the economic injury level, allowing time for control measures to take effect. The treatment threshold can vary depending on the abundance of a pest’s natural enemies. Higher densities can be tolerated when populations of natural enemies are also high. For example, the treatment threshold for an insect might be 3 per leaf if there are no natural enemies but 6 per leaf if predators or parasites are abundant.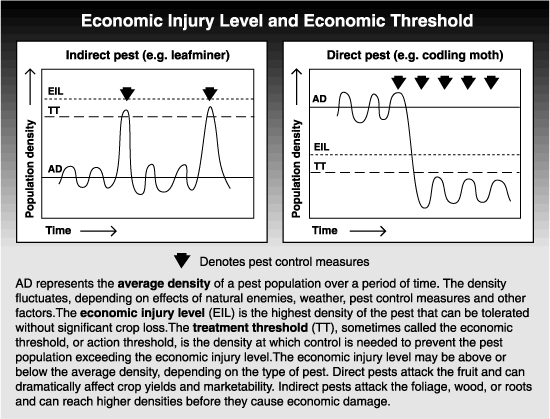
Management tactics
A variety of management tactics can be used to prevent a pest population from exceeding the economic injury level. Integrated pest management stresses reliance on control methods that will be the least disruptive of natural enemies while still providing adequate control of pests.
Synthetic organic insecticides have been the dominant pest control tactic used in orchards since their introduction soon after World War II. There are four main groups: organophosphates, organochlorines, carbamates and pyrethroids. They have been relatively inexpensive, highly effective and fast acting, often providing almost complete control. They affect a wide range of organisms and often kill natural enemies as well as pests. In addition, many pests have developed resistance to them. These powerful pest control tools should be used only as a last line of defense against pests. Natural enemies, selective insecticides and other nondisruptive control tactics should be given every opportunity to work before broad-spectrum insecticide are used.
Insect growth regulators (IGRs) are synthetic chemicals that mimic or inhibit natural hormones that govern an insect’s development (see section on Insect Growth Regulators). When exposed to these chemicals, the insect develops abnormally and dies. Insect growth regulators have been an important component of IPM programs in Europe since the 1980s, but none has been registered in the United States for use on fruit crops.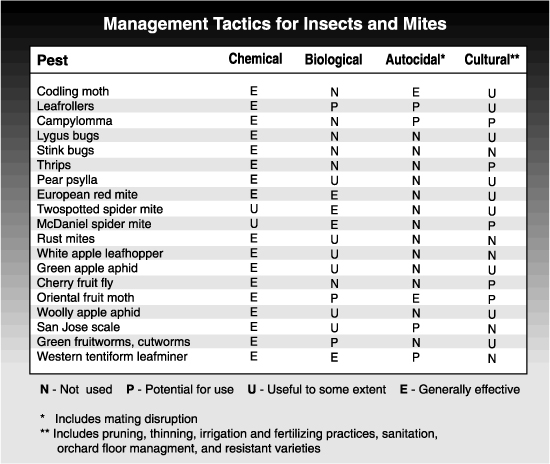
Botanical insecticides are derived directly from plant or plant products. The three botanicals most frequently used to control orchard pests are ryania, pyrethrum and rotenone. Ryania is extracted from the roots of Ryania speciosa, a shrub grown in tropical America. Its use in orchards has been primarily against lepidopteran larvae, particularly codling moth. Pyrethrum is extracted from flower petals of certain Chrysanthemum species and has a fairly wide spectrum of activity against insects. Rotenone is obtained from the roots of certain species of leguminous shrubs grown in Malaysia, the East Indies, and South America. This insecticide can be used to control some chewing and sucking insects. Since botanicals are fairly expensive and generally less effective or have a shorter residual effect than synthetic organic insecticides, they are used primarily in production of organic fruit.
Insecticidal soaps are available to control soft-bodied pests such as pear psylla, aphids, scales, leafhoppers and mites. Applications must be timed precisely to coincide with the pest’s most susceptible stage and applied frequently for greatest success. Soaps may have phytotoxic effects on some crops. The major advantage to using soaps is their low toxicity to natural enemies, as well as humans.
Diatomaceous earth is finely milled fossilized shells of fresh water diatoms. The microscopic silica dust is damaging to soft-bodied insects such as aphids and pear psylla. It is thought to kill them by physically damaging their membranes, leading to a loss of body fluids.
Lime-sulfur and wettable sulfur are used to control fungal diseases and mites, scale insects, aphids and pear psylla. Both formulations are incompatible with many other spray materials and if used improperly can be phytotoxic. Sulfur is toxic to predaceous mites and can be disruptive to integrated mite control programs if used in summer.
Horticultural spray oils are produced by distilling and refining crude petroleum oils. They are often used in combination with other chemicals but can be used alone to suppress insects and mites. Applied during the dormant or delayed dormant period, they kill overwintering stages of pests such as scales and eggs of European red mite. They also inhibit egg laying by pear psylla females. To date, there are no documented cases of an insect developing resistance to oils. The mode of action is not clear, but several mechanisms are suspected including smothering the insect or its eggs or penetrating the insect’s cuticle and interfering with nerve transmission. Oils can be phytotoxic to fruit and foliage of deciduous tree fruits. Damage varies with the type of oil (paraffin content, unsulfonated residue, distillation range, viscosity), the tree species, application concentration, and the weather before, during or after the application. Combining oil with certain pesticides or nutrients may exacerbate phytotoxicity.
Microbial insecticides are developed from insect pathogens such as viruses, bacteria or fungi. They have several advantages over traditional pesticides. They are more selective, usually not toxic to predaceous or parasitic insects, and have a much less harmful effect on the environment. The bacterial insecticide Bacillus thuringiensis (Bt) is effective against lepidopteran larvae such as leafrollers, peach twig borer and cutworms. Bt is not a contact insecticide and must be consumed to be effective. When ingested, the Bt produces a biotoxin which makes holes in the insect’s stomach lining. Bacterial spores then get into the insect’s blood stream and poison it. Once it has consumed a toxic dose, the larva stops feeding but may remain alive for several days. Bt is most effective against young larvae, as it takes a smaller dose to kill them than it takes for more mature larvae. Bt will kill codling moth larvae in the laboratory, but because they are exposed for such a short time before entering the fruit (probably not long enough to consume a lethal dose) it is not a highly effective field control. The codling moth granulosis virus is a highly selective microbial insecticide. To be effective, it must be eaten by larvae just after they hatch so sprays must be applied frequently. Both Bt and viruses have a short effective life of 3 to 7 days. They break down in sunlight and must be applied more frequently than traditional insecticides to achieve adequate control. Because larvae must consume these products, thorough coverage is essential.
Biological control is a pest control tactic where a pest’s natural enemies (predators and parasitoids) are used to keep its density below damaging levels. Every pest has natural enemies, but whether they can provide adequate control in an orchard is difficult to predict. To make the most effective use of biological control you must know the life cycle, biology and ecology of both the pest and its natural enemies. Biological control alone will not be sufficient to control all pests attacking a tree fruit crop, but it plays an important part in an IPM program. Biological control can either occur naturally or can be encouraged by introducing natural enemies into the orchard. It also can be enhanced by protecting natural enemies that are in the orchard and providing suitable habitat near the orchard or in the cover crop to promote their survival. Natural enemies have the potential to control many secondary pests such as mites, aphids, leafminer, pear psylla and grape mealybug. Broad-spectrum insecticides kill most natural enemies, allowing outbreaks of pests they would normally control. Biological control is relatively safe, often permanent and economical. However, it can take time to implement a biological control program, and it may mean having to tolerate some crop injury until natural enemies build up to sufficient numbers to provide adequate control (see also section on Biological Control).
In mating disruption, pheromones are used to prevent male insects from finding females. When used in place of broad-spectrum insecticides for control of key pests, such as codling moth, it allows improved biological control of many indirect pests and slows the development of insecticide resistance. Pheromones are highly specific, affecting only the target pest, and are not toxic to mammals in the amounts used for mating disruption. They leave no residues on the crop and have no adverse impact on the environment (see also section on Mating Disruption).
Cultural controls are used to some extent for most apple pests. However, few provide complete control of any pest on a regular basis. They are used most often to reduce the potential for pest development and are combined with other control tactics. Cultural controls include:
- Pruning out water sprouts, sucker growth or foliage preferred by insects such as aphids, pear psylla and leafhoppers
- Sanitation, including the removal and destruction of infested fruit and pupation sites of key pests, such as codling moth
- Pruning practices that eliminate optimum sites for mealybug and scales and which enhance penetration of sprays
- Planting high density orchards with trees that have relatively smooth bark and easily sprayed canopies
- Managing fertilizer to limit the excessive plant growth preferred by plant-sucking insects
- Eliminating pests’ alternate plant hosts in or around the orchard
- Maintaining a ground cover that provides habitat for beneficial insects