by Mike Doerr and Jay F. Brunner, published online December 2007
(Lepidoptera: Noctuidae)
Lacanobia subjuncta is native to Washington but was not considered a pest in Washington State orchards until recently. This fruitworm feeds on a wide variety of native plants and agricultural crops. During the mid-1990s, L. subjuncta was reported infesting apple orchards in the Columbia Basin. When infesting fruit trees the larvae feed primarily on foliage and can defoliate entire growing shoots. Mature larvae can also cause significant levels of fruit injury when present in high densities.
Hosts
Lacanobia subjuncta occurs throughout North America and is reported to feed on row crops, shrubs, trees and several weed species that are found in orchard ground covers (i.e., dandelion, bindweed, mallow). Larvae are primarily a pest of apple but have also been reported feeding on pear.
Life Stages
Egg
Eggs are laid on the underside of fruit tree leaves. The shape of the eggs is typical of fruitworm. They are pale gray when laid and turn a slightly purple color as they mature. The egg is spherical with a slightly flattened top and has vertical ribs extending from a micropyle on the top of the egg to the equator. Eggs are loosely grouped together in masses of approximately 100.
Larva
The larva is the most distinctive stage of L. subjuncta. Both its color and pattern change as it grows. Earlier instars resemble green fruitworms (green with a lateral stripe). The color of later instars ranges from bright green to tan to a light red or brick color. It is the mature larva that displays the characteristic herringbone pattern on its dorsal side. The last instar larva is large, nearly two inches in length.
Pupa
L. subjuncta pupates in the soil near the host plant. The pupa is dark brown and naked, that is, not found within a silken cocoon.
Adult
The L. subjuncta adult is about 1 inch (25 mm) long and 2 inches (50 mm) between the wing tips. L. subjuncta has a distinctive color pattern of scales on the wing that range from light brown to black. The reniform and orbicular spots are light brown with a black border. These spots can be easily seen and help to identify this species from other Noctuids
Life history
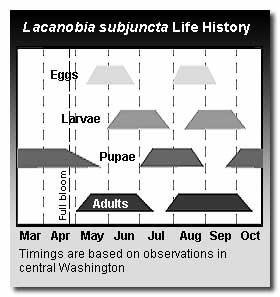 There are two generations per year on apples in Washington. L. subjuncta overwinters as a pupa in the soil and adults emerge in May and June. Oviposition begins in mid-June and lasts for about six weeks. Eggs may be deposited in fruit trees or on weed hosts. First generation larvae are present in June and July. Though these larvae feed primarily on foliage, significant fruit injury can also occur at this time. Unlike other noctuid pests of apples, which generally move between the tree and the ground cover, larvae of this fruitworm can remain in the tree to complete larval development. Larvae are voracious feeders and can defoliate shoots in a few days. The emergence of second generation adults begins in late July and they are active through October. Larval feeding from the second generation begins in mid-August and larvae can be found into October.
There are two generations per year on apples in Washington. L. subjuncta overwinters as a pupa in the soil and adults emerge in May and June. Oviposition begins in mid-June and lasts for about six weeks. Eggs may be deposited in fruit trees or on weed hosts. First generation larvae are present in June and July. Though these larvae feed primarily on foliage, significant fruit injury can also occur at this time. Unlike other noctuid pests of apples, which generally move between the tree and the ground cover, larvae of this fruitworm can remain in the tree to complete larval development. Larvae are voracious feeders and can defoliate shoots in a few days. The emergence of second generation adults begins in late July and they are active through October. Larval feeding from the second generation begins in mid-August and larvae can be found into October.
Damage
Newly hatched L. subjuncta larvae feed in a clustered group near the egg mass. Young larvae feed on the lower leaf surface removing most of the green leaf tissue between leaf veins. The upper epidermis of the leaf is left in tact creating a window-pane effect. As larvae mature, they disperse and generally move downward from the egg mass in a cone shaped distribution. Evidence of feeding damage corresponds to this general pattern of movement. It appears that a majority of larvae leave the trees in favor of feeding on ground cover; however, a significant number do remain in the tree causing further injury.

As larvae mature, the feeding is more typical of a fruitworm than a cutworm. The term “cutworm” refers to larvae that bite at the stems of plant tissue causing them to fall over. L. subjuncta larvae feed on leaf tissue first causing holes in the leaf interior then on the periphery and then eating the entire leaf. Fruit injury is incidental to foliage feeding but can be quite severe in orchards where the densities are high. More fruit damage can occur where clustered fruit is in close proximity to dense foliage or tall growing weeds, such as the lower center of apple trees. Larvae feed directly on fruit by excavating holes. These holes can be as large as a fingertip. As larvae grow they are very mobile within the canopy and feeding damage becomes evenly distributed.
Biological Control
Small numbers of parasites have been reared from field-collected L. subjuncta larvae. Although not yet identified to species, these include several hymenopterous parasites and at least 3 species of Tachinidae. L. subjuncta attacked by tachinid flies can be recognized by the white or cream-colored egg attached usually to the head of the larvae.
Management
Monitoring
Three methods have proven effective in monitoring L. subjuncta populations. A general purpose bucket-style trap baited with a sex pheromone is useful for monitoring adult males, a limb tap/beating tray technique is useful as a direct sampling method for larvae , and visual examination of foliage for signs of feeding damage provides a good indicator of larval presence.

The pheromone lure is very attractive and large numbers of moths may be captured in orchards containing few larvae. In order to limit the effect of outside populations biasing trap data, traps should be placed in the center of each orchard block of 10 acres or larger.
Direct sampling of larvae using a limb tap/beating tray method requires a large number of samples to accurately measure populations. At least 25 beating tray samples spread throughout a large block is necessary to obtain an accurate measure of L. subjuncta density. Larvae are more likely to be dislodged where foliage is the densest; therefore, sampling should be concentrated in the lower interior of the trees. The beating tray sampling technique is biased towards younger larvae so this sample is most reliable when the majority of eggs have hatched and larvae are still in the early instars (see Degree-Day Model section below).
The most reliable measure of L. subjuncta density is a visual inspection of foliage feeding. In order to optimize pesticide programs, it is best to conduct this sample when egg hatch is nearly complete and before larvae become too large. Visual inspection also requires that a large number of trees be examined because larvae are not uniformly distributed throughout an orchard. Twenty shoots from 25 trees should be examined in a large block (10 to 20 acres) noting the percentage of shoots that show feeding damage.
Thresholds
There is not a good correlation between any measure of L. subjuncta density or foliage feeding activity and fruit injury. This is most likely because L. subjuncta only incidentally feed on fruit and horticulture factors like crop load, clustering of fruit, shoot growth, weed management and fruit maturity can all influence the amount of fruit damage that occurs.
Apple orchards can tolerate relatively high L. subjuncta densities before significant fruit injury occurs. Although the correlation between assessments of larval feeding activity and fruit injury are not strong, there are some general guidelines that can be followed. When 30-35% of shoots show feeding damage then about 1% fruit injury can be expected. This threshold is based on the amount of foliage feeding present at 100% egg hatch , a time that is a little too late for optimized insecticide applications that target young larvae. Therefore, treatment thresholds based on trap catch or beating trays are needed to predict either fruit injury or shoot infestations.
Peak trap catch provides an indicator of population level in an area and can serve as a presence/absence indicator. Typically, orchards with peak trap catch less than 150 moths/week did not have shoot injury that would exceed the 30-35% shoot feeding threshold. The main problem in using peak trap captures as a threshold is that some orchards will have very high peak trap catches but have low levels of foliage feeding. Therefore, a peak trap catch that exceeds 150/week should trigger a search for egg masses in late May-early June and/or use of beating tray samples and foliage inspection in mid-June to further assess L. subjuncta densities. If egg masses or larvae are not detected in-orchard then controls would not be required. A sample of neighboring blocks or areas containing weeds such as mallow, curly dock, dandelion, pigweed or lambs quarter may reveal the external source of moths. What constitutes a treatable population is one of the most important decisions for managing L. subjuncta. However, at this time there is no single density measure will provide an accurate and reliable prediction of the risk of fruit injury from this pest.
Degree-day Model
With little detailed information on a pest’s phenology, sampling for larvae and timing insecticide applications is relegated to a a trial and error exercise. The result has often been an increase in rates and frequency of pesticide applications. This increases grower costs as well as the potential for negative impact on biological control activity in the system.
A degree-day model has been designed to identify important periods in L. subjuncta phenology through the growing season. The lower and upper thresholds for L. subjuncta are 44 and 90ºF. A horizontal cutoff is used when calculating degree-days using maximum and minimum temperatures beginning March 1. A degree-day look-up table for L. subjuncta and a degree day development phenology table are available.
 The detailed phenology descriptions presented here are expected to make the timing of insecticide applications more accurate and therefore more effective. For example, the ideal timing for the application of a “selective” pesticide with relatively short residual activity is when the majority of larvae are of a susceptible stage – third instar or earlier. Therefore, control sprays should be applied by 1230-1250 degree-days, when only about 10% of the larvae are in their 4th instar. This time represents the best opportunity to control L. subjuncta with a single insecticide application.
The detailed phenology descriptions presented here are expected to make the timing of insecticide applications more accurate and therefore more effective. For example, the ideal timing for the application of a “selective” pesticide with relatively short residual activity is when the majority of larvae are of a susceptible stage – third instar or earlier. Therefore, control sprays should be applied by 1230-1250 degree-days, when only about 10% of the larvae are in their 4th instar. This time represents the best opportunity to control L. subjuncta with a single insecticide application.
During the second generation, 10% of the 4th instar are estimated at 3050 degree-days. The best timing for an insecticide application against larvae of the second generation is at 3050 degree-days, but no later than 3150 degree-days.
Another use of the model is to provide growers and crop consultants with a tool to schedule a larval sampling activity just prior to the time for an optimum insecticide application. By sampling just prior to the windows discussed above an estimate of the number of larvae or percent shoot feeding can be made to ascertain the need to apply a control.
Chemical Control
Materials available for apple
Excerpt from the WSU Crop Protection Guide. For timings at which each pesticide can be used refer to the Crop Protection Guide.
YOU ARE REQUIRED BY LAW TO FOLLOW THE LABEL. It is a legal document. Always read the label before using any pesticide. You, the grower, are responsible for safe pesticide use. Trade (brand) names are provided for your reference only. No discrimination is intended, and other pesticides with the same active ingredient may be suitable. No endorsement is implied.
Articles from the Tree Fruit website may only be republished with prior author permission © Washington State University. Reprint articles with permission must include: Originally published by Washington State Tree Fruit Extension at treefruit.wsu.edu and a link to the original article.



