by Timothy J. Dennehy and John Dunley, originally published 1993
Though it has been over 80 years since the first discovery of a major agricultural pest becoming resistant to a pesticide, it was not until the 1950s that most growers became familiar with pesticide resistance as a result of the widespread development of insect resistance to DDT. Since then, growers have come to expect the eventual loss of pesticide effectiveness because of resistance. By the mid-1980s, there were records of about 450 resistant species of insects and mites.
What is resistance to pesticides?
It is essential to distinguish between two common but very different contexts in which the word “resistance” is used. Specifically, it is important to understand how resistance is defined in the scientific context versus how it is manifest in the field.
Resistance as defined in the laboratory
Resistance, from the scientific perspective, is a heritable, statistically defined decrease in sensitivity to a chemical in a pest population relative to the response of susceptible populations that have never been exposed to pesticides. Resistance can be demonstrated by comparing, in laboratory tests, differences in susceptibility between a susceptible population and a population that can withstand to some degree the effects of a pesticide. Evidence of resistance in the laboratory does not necessarily mean that a chemical will fail in the field. Pesticide effectiveness is influenced by several factors, and resistant pests may or may not be adequately controlled by the insecticide given a set of treatment conditions (rate, volume, coverage, etc.). However, the fact remains that resistant pests can be demonstrated to defeat the toxic action of a pesticide to a greater degree than susceptible pests.
Resistance as manifested in the field
Resistance that is manifest in the field results in measurable reductions in the relative efficacy of a pesticide. Estimating the impact of this reduction of control is an important step in managing resistance. To do this, conventional spray trials are replicated, with the same application equipment and conditions used by growers, at field locations with different levels of resistance.
When resistance reduces the relative efficacy of a pesticide, the chemical will provide significantly less control of the pest at locations with higher frequencies of resistance than at locations with lower frequencies or no resistance. Because field trials usually do not reveal the development of resistance in its early stages, by the time field personnel notice that pesticide performance is declining, resistance often has built up to fairly high frequencies in populations. It is for this reason that information from laboratory tests, rather than field experience, must be used for early detection of resistance.
Loss of relative efficacy of a chemical is not necessarily proof of resistance. For example, some instances of poor pesticide performance, initially attributed to pest resistance, have proved to be caused by a breakdown of the pesticide by soil microorganisms or high pH of spray water or by poor pesticide application procedures.
Mechanisms of resistance
There are two common mechanisms by which insects and mites overcome the toxic action of pesticides, increased metabolic detoxication and decreased target site sensitivity. Resistant pests with enhanced metabolic detoxication are able to disarm toxic pesticide molecules more rapidly than susceptible individuals. As a result, less of the active pesticide sprayed on the field reaches the target site in the pest.
With the second common mechanism, decreased target site sensitivity, the physiological target for the pesticide in the resistant insect is less sensitive to poisoning than in susceptible individuals. For example, organophosphate insecticides kill pests by inhibiting acetylcholinesterase, an enzyme that is important in nerve function. Some insects, mites, and ticks resistant to organophosphates have a form of the target enzyme that is less sensitive to poisoning.
Resistance also can be enhanced by reduced cuticular penetration of the pesticide, though this appears to be a less common mechanism than those above. Reduced penetration alone generally results in only low intensities of resistance, but when combined with increased metabolic detoxication or decreased target site sensitivity it can result in very intense resistances. A little-studied but potentially important resistance mechanism involves changes in pests’ behavior. It is likely that some behavioral resistances enable pests to reduce contact with pesticides on treated plants.
How Resistance Develops
Resistance develops via the process of selection by a chemical on the genetic variation in susceptibility within a pest population.
How selection happens is easy to understand. At first, only a very small proportion of a pest population can survive exposure to the pesticide, but each time the pesticide is applied, a greater proportion of resistant individuals survive than susceptible types. These resistant individuals pass the genes for pesticide resistance to their progeny. Each use of the pesticide increases the proportion of the less-susceptible individuals in the population.
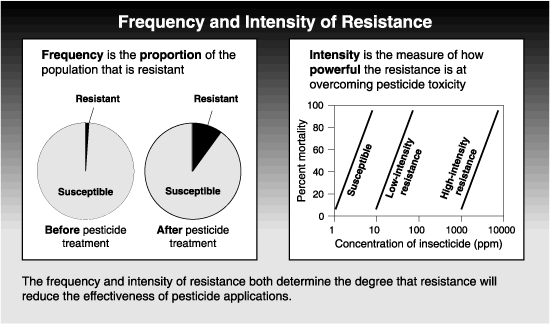
The degree to which resistance reduces the relative efficacy of a pesticide depends on both the frequency and the intensity of the resistance. Frequency refers to the proportion of the pest population that is resistant ; intensity is the strength of the resistance in each resistant pest. Obviously, resistance is more likely to become a problem as the frequency of resistant individuals increases in a population. But the intensity of a resistance can also affect the pesticide’s efficacy in the field. In some cases, control of the resistant pest is only slightly affected by resistance. In other cases, the pest becomes virtually immune to the pesticide.
A pest population may have a resistance of low intensity at a high frequency (proportion of the population) without significantly affecting field performance of the pesticide. On the other hand, a very intense resistance might reduce the efficacy of a chemical, even when present a low frequencies in a pest population.
Cross resistance and multiple resistance
In nearly half the recorded cases of resistant insects and mites, the pests are resistant to between two and five different classes of chemicals. Pests that are resistant to many pesticides pose an especially difficult problem when chemical control is required. Understanding the distinction between multiple resistance and cross resistance is important in order to grasp the practical ramifications of pests having more than one resistance factor.
Cross resistance
Many resistances are conferred by a single major genetic factor that differs between resistant and susceptible pests. When a single factor confers resistance to more than one pesticide, this is cross resistance. For example, a mechanism making insects resistant to parathion also dramatically reduces susceptibility to a number of other organophosphates. Therefore, when parathion becomes ineffective due to resistance, some other organophosphates also lose efficacy because of cross resistance. The key point is that with cross resistance a single mechanism is responsible for resistance to more than one pesticide, and cross resistance can cause resistance to build up to pesticides that you may never have used. Cross resistance also means that other classes of pesticides, e.g. organophosphates and carbamates, would be less effective where resistance occurs to either.
Multiple resistance
With multiple resistances, two resistance mechanisms are acquired independently through exposure to two different pesticides. For example, some spider mites possess resistance to cyhexatin (Plictran) and dicofol (Kelthane). However, these resistances are acquired by two separate genetic modifications, and resistance of the pest to one product does not affect susceptibility to the other. When a pest is resistant to two pesticides the only way to find out if it is due to cross resistance or multiple resistance is by conducting genetic studies in the laboratory.
Resistance stability
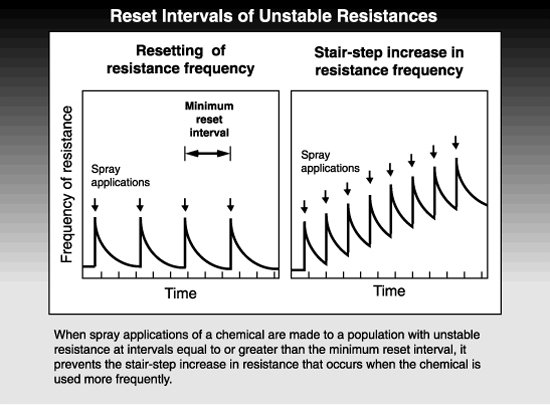
Resistances can be either stable or unstable in the field, depending upon many factors involving the pest, the chemical, and the agricultural system used. A stable resistance increases in frequency when a pesticide is used and does not decline appreciably thereafter. An unstable resistance similarly increases in response to pesticide treatments but decreases in frequency during intervals when the pesticide is no longer used.
The phenomenon of unstable resistance can be exploited to manage resistance. Under a given set of circumstances, research can be conducted to estimate the length of time needed for the frequency of resistant pests to be reset, i.e., to decline to levels existing before the last treatment was applied. Then a resistance management program can be developed that employs pesticide rotations that allow for this resetting of resistance. The shortest interval in which the same chemical can be used and still allow resistance frequencies to reset is called the minimum reset interval. If treatments are spaced at less than the reset interval, the resistance increases in a stair-step fashion, but if the interval between treatments is as long or longer than the reset interval, there will be no net increase in resistance.
Reset intervals will vary between crops and geographic locations. Once an appropriate reset interval has been established for a system, appropriately selected pesticides can be used in rotation to maintain their efficacy and resistance can be monitored to evaluate the success of the program.
Factors influencing resistance
Researchers have shown that resistance development in pest populations is influenced by many biological, ecological, genetic, and operational factors.
Biological and ecological factors include:
- Characteristics of the pest, such as the rate of reproduction, the number of generations per year and mobility of the species
- Characteristics of the orchard, such as proximity to untreated areas, suitability of alternate hosts for pest development, immigration of susceptible pests and effectiveness of biological control
Genetic factors include:
- The number of genes conferring resistance
- The frequency and intensity of resistance genes in the population
- The ability of resistant individuals to grow and reproduce relative to susceptible pests
Operational factors include:
- Characteristics of the chemical
- Treatment thresholds
- Application methodology and equipment
- Chemical use strategies such as chemical rotations or mixtures.
In practice, many of these factors are not readily manipulated by growers. From the practical standpoint, growers wishing to manage resistance should give attention to the following factors:
- How effectively you employ methods of integrated pest management
- How often you use pesticides
- How you select and apply the pesticides you use
Growers who use pesticides the least have the most effective resistance management programs. Resistance cannot be managed in situations where a pesticide is used many times each season.
Chemical use strategies for resistance management
Since selection and use of pesticides are variables that growers can normally manipulate, identifying optimal chemical use recommendations is a critical step in building a resistance management program. Chemical use recommendations are based on one of three different strategies:
- Management by moderation
- Management by multiple attack
- Management by saturation
Management by moderation is probably the most universal principle for successfully managing resistance. It involves reducing overall chemical use or persistence by:
- Using lower dosages of pesticides (when appropriate);using higher treatment thresholds
- Using chemicals with shorter residual activity
- Treating only limited areas in orchard
- Maintaining unsprayed areas as refuges for susceptible individuals
- Spraying only specific pest stages
Many of these approaches are already common components of IPM programs. Practices that protect and promote natural enemies contribute to resistance management since they reduce pesticide use. In addition, natural enemies consume resistant and susceptible individuals indiscriminately, thus helping counteract pesticide selection.
Management by multiple attack involves using either mixtures or rotations of pesticides to thwart resistance. Use of mixtures, for example tank mixes of two or more pesticides, is based on the concept that insects resistant to one pesticide will be killed by the other component(s) of the mixture and that few pests will be resistant to the entire mixture.
Though mixtures of fungicides have been used for years to combat resistance, both field experience and models have shown that mixtures should be avoided whenever possible with insects and spider mites. Commonly, use of mixtures of insecticides or acaricides has resulted in pest populations developing high frequencies of resistance to all pesticides in the mixture, an outcome with disastrous consequences for IPM programs. By far the safest approach is to rotate insecticides or acaricides so that each product is used as seldom as possible in any given season.
Management by saturation involves methods that overcome resistance mechanisms present in pests. The most common method is to combat resistance by using high rates of pesticides, ones that kill even resistant individuals. Deceptively reasonable on first inspection, this approach rarely works in practice. The reason is that pesticide residues are usually deposited very unevenly in most field situations, even when very high rates are used. Uneven deposition of pesticides allows resistant pests to survive in greater proportions than susceptible pests, thereby increasing resistance. In addition, use of high rates can have many detrimental impacts on natural enemies, the environment, and human health.
A completely different application of the management by saturation concept involves using chemical synergists, products that enhance the action of pesticides. Some synergists have been used successfully to neutralize metabolic pathways conferring resistance.
Proactive versus reactive resistance management
In the past, resistance studies typically have been undertaken or supported by chemical manufacturers only after a pesticide has been used for many years and failures of the product in the field have become commonplace. This is reactive resistance management. Resistance is known or strongly suspected because of repeated reports of product failure, and the initial research objectives are to develop ways to monitor resistance, assess its intensity and frequency in field populations, and characterize the biochemical mechanism of resistance. Thereafter, field studies focus on understanding how quickly resistance builds up following chemical treatment and how fast, if at all, it declines in the absence of the pesticide.
In recent years, progressive chemical manufacturers have not waited until their products have failed before initiating resistance management efforts. Some producers are now addressing management of resistance from the very early stages of product development. They are collecting baseline susceptibility information from populations collected from around the world, looking for potential resistance problems, and proposing provisional resistance management strategies for their products early in the process of development. These proactive resistance management efforts reflect an overall increase in the sophistication of the chemical industry’s resistance management efforts and are likely to prove very beneficial.
Resistance management as a component of IPM
Pesticide resistance management will extend the useful life of valuable IPM-compatible pesticides. Yet, resistance management is likely to be successful only where orchardists:
- Routinely monitor pests
- Use reasonable treatment thresholds
- Make full use of nonpesticidal methods, such as biological and cultural control, sanitation and host plant resistance.
For well developed IPM systems, like those of Pacific Northwest tree fruit, researchers have made considerable progress toward putting in place the essential components of resistance management programs. For example, the efficacy of acaricides for mite control in the Pacific Northwest has been sustained for long periods of time where they were used as part of IPM programs.
The lack of registration of new pesticides, coupled with a loss of registered pesticides to the regulatory process or to resistance, will leave growers with few or no registered products that adequately control key pests. It is unrealistic to believe that we can successfully manage resistance if pesticides are not used sparingly. Experience from the Pacific Northwest and elsewhere demonstrates that with good IPM practices, the efficacy of key pesticides can be prolonged considerably and, in some cases, maintained indefinitely.