Written by: Tianna DuPont, WSU Extension; Scott Harper, WSU Plant Pathology; Tobin Northfield, Louis Nottingham, WSU Entomology, Adrian Marshall, Rodney Cooper, USDA-ARS TTFVRU; Corina Serban, Bernardita Sallato, WSU Extension; Last updated September 2025. Peer reviewed FS401E
The X-disease phytoplasma (Candidatus Phytoplasma pruni) is the primary cause of small, pale, tasteless, and unmarketable cherries in Washington State (Figure 1 and Figure 2). In peaches, plums, and nectarines, X-disease symptoms are typically yellowed, curled, and shot-holed leaves, and small, deformed fruit (Figure 3). X-disease is present across North America, widespread throughout Washington State, and occurs at high incidence in Yakima, Benton, Franklin, Grant, and Chelan Counties. It is also present in Douglas, Okanogan, Klickitat Counties, and in Oregon in The Dalles area.
Background
X-disease also known as Western X, Cherry Buckskin, and Peach X, is not a new disease, but Washington is experiencing new complications. It was first identified in Washington State cherry trees in 1946. In a 1947 survey, about 1% of cherry trees were found to be infected, and it has remained present ever since, fluctuating in frequency. However, it has increased in prevalence dramatically in recent years, with pathogen strains, vectors, and environmental conditions that were not present in the previous outbreak.
Symptoms
Infection reduces fruit size and quality in sweet cherries. In contrast to little cherry virus 2 where fruit are often bitter, fruit from X-disease infected trees are often tasteless. Fruit from X-disease infected trees have reduced fructose, glucose, and sorbitol content and in some cases total phenolic content increases (Wright et al. 2021; Harper et al. 2020). The primary strains present in the Pacific Northwest do not generally express foliar symptoms on cherry, whereas strains in other parts of the country can produce enlarged styles and witches’ broom.
Symptoms in cherry
- Small and misshapen fruit.
- Poor color development.
- Fruit bitter or lacking flavor.
- Symptoms can be confused with unripe fruit until close to harvest.
- Symptoms are restricted to one or a few branches unless trees have been infected for multiple years.
Symptoms in peaches, plums, and nectarines
- Yellowed, curled leaves.
- Leaf shot hole.
- Small, deformed fruit.
- Leaf yellowing symptoms on infected peaches and nectarines begin to appear about two months prior to harvest, and get progressively worse, with shot holes appearing as the season progresses.
Symptom progression
- Years 1 and 2: Small fruit may be restricted to one branch or cluster. Fruit color may develop normally, or individual pale to white fruit may be observed. Early infections may display no notable symptoms.
- Years 3–4: Systemically infected tree. Small fruit observed on multiple or all limbs, and poor color development is pronounced.
- 5+ years: Cultivar dependent, but characterized by reduced fruit yield and dieback of limbs.
Causal Organism
X-disease phytoplasma is not a virus but instead is a type of wall-less bacterium known as a phytoplasma. The X-disease phytoplasma lives and replicates in the vascular phloem of infected trees, interfering with tree growth and development. Five major strains of Ca. P. pruni have been found to cause X-disease in commercially grown Prunus species (Molnar et al. 2024). These strains differ in geographic distribution, severity, symptoms, and vector transmissibility. Unfortunately, the dominant strains in the Pacific Northwest cause fruit symptoms, but not leaf symptoms or stunting, making them more difficult to scout for.
Occurrence
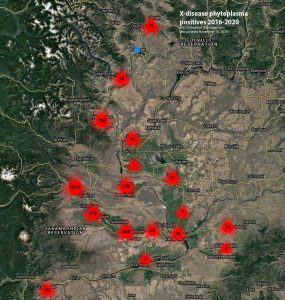
X-disease is present across North America and throughout Washington State, with high incidence in Grant, Yakima, Benton, Franklin, and Chelan Counties, and is also present in the Oregon counties of Okanogan, Douglas, and Klickitat as well as in The Dalles area (Figure 4) (Molnar et al. 2022).
Impacts
More than 238,856 sweet cherry trees (covering 974 acres) and 33,082 peach, nectarine, plum and apricot trees (covering 81 acres) were removed due to X-disease and little cherry disease (caused by LCV2 and LCV1) between 2015 and 2020 according to a recent survey conducted by Washington State University Extension and Oregon State University Extension. The survey included 81 respondents who collectively manage 15,420 acres, 26% of the total cherry acreage in Washington and Oregon (Molnar et al. 2022). Tree removals reduced industry revenue by an estimated $30 million in 2020 and $65 million between 2015 and 2020. Over the seven-year re-establishment period, estimated lost revenue and establishment costs to growers were approximately $115 million.
Host Range
X-disease phytoplasma infects most Prunus species (e.g., cherries, peaches, nectarines, almonds, plums, and chokecherry). X-disease phytoplasma also infects a wide range of broadleaf plants. Previous studies found milkweed, alfalfa, clover, mustards, and plantains to be hosts (Jensen 1971; Chiykowski and Sinha 1982), but this was mostly through experimental inoculation. To identify the natural host range, a survey was conducted from 2021 to 2023 in which a total of 52 plant species, from 77 species tested, were positive for X-disease phytoplasma (Figure 5) (Shires et al. 2024). Plants from six families had significant numbers of positives, including members of the Asteraceae (dandelion), Malvaceae (mallow), Amaranthaceae (goosefoot/lambsquarter and pigweed), Polygonaceae (knotweed), Brassicaceae (flixweed, tumble mustard, hoary bittercress, and shepherd’s purse), and Plantaginaceae (plantain). All these species are common components of the orchard floor or are present around orchard borders. Twelve other plant families were less prevalent in orchards and had a small number of positives for X-disease phytoplasma, including Apiaceae (e.g., queen anne’s lace and wild carrot), Caryophyllaceae (chickweed), Fabaceae (clover), Geraniaceae (redstem filaree), Lamiaceae (henbit, purple dead nettle), Oxalidaceae (oxalis, ground sorrel), Poaceae (downy brome), Rosaceae (wild rose), Solanaceae (nightshades), and Tribulus (puncture vine).
Transmission
Grafting
X-disease phytoplasma is readily transmitted by all types of grafting.
Vector
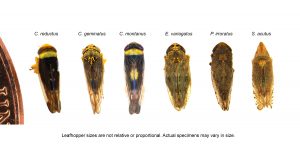
Leafhoppers are the only known vectors. Seven leafhoppers are known to transmit X-disease phytoplasma: Colladonus reductus, C. geminatus, Euscelidius variegatus, C. montanus, Fieberiella florii, Scaphytopius acutus, and Paraphlepsius irroratus (Figures 6 and 7) (Jensen 1969; Purcell and Elkinton 1980). In tests of over 2,000 individuals, no other leafhoppers were found positive for X-disease phytoplasma (Northfield et al. 2023). The only species found actively transmitting in that survey were C. reductus and C. geminatus. C. reductus is the most common X-disease leafhopper vector in Washington, followed by E. variegatus and, at a lower frequency, C. geminatus, although relative abundances differ by location. Low numbers of S. acutus were found in Washington surveys from 2021 to 2022 (Northfield et al. 2023; Northfield and Nottingham 2020). Research in the 1960s suggests that E. variegatus is a less effective vector than C. reductus, with a longer latency period (approximately 50 days compared to 30–35 days) (Jensen 1969). This study’s results align with our finding that field-collected E. variegatus never had enough phytoplasma in their mouthparts for transmission (Marshall et al. 2025). Leafhopper vector transmission will vary depending on the level of infection in the block as well as the numbers of leafhoppers. For example, in a two-year study of managed orchards with some X-disease incidence, 9%–25% had acquired the phytoplasma, and up to 1.3% of Colladonus spp. leafhoppers were able to transmit it (Northfield et al. 2023). However, in an unmanaged orchard with high levels of infection (50 of 50 trees testing positive), 40% of C. reductus leafhoppers had acquired the phytoplasmas (Marshall, unpublished data).

Life Cycle of the Organism
The X-disease phytoplasma lives and replicates in the phloem tissues of the tree. Over the winter, the phytoplasma numbers in the aerial parts of the tree decline but do not die out completely, while higher levels of phytoplasma survive in the roots. In the spring, the aerial portions of the tree become re-infected as the phytoplasma moves through the phloem of the tree following source-to-sink pathways. As a result, you may see symptoms in one limb for a year or more, but symptoms will eventually appear in additional limbs. Removing a symptomatic limb does not eliminate the phytoplasma since it is already in the root system before symptoms appear.
Vector Biology
In Washington, C. reductus has three periods of peak adult abundance: early summer (June), late summer (August), and early fall after harvest (September and October), indicating a potential for three generations per year (Marshall et al. 2024). In California, C. reductus was found to have a generation time of 56 days (Severin and Klostermeyer 1950). C. reductus feed on a wide range of hosts according to feeding trials, including mallow, alfalfa, cherry, peach, white clover, and dandelion (Northfield and Cooper 2020). Current phenology of C. geminatus is largely unknown due to low abundance in recent years. Research in the 1940s and ’50s suggested it had generation times of 60 and 56 days in Oregon and California, respectively, with adults emerging in May and September in Oregon (Nielson 1968), which is generally supported by recent research.
Sampling and Testing for X-disease
Material to sample:
Submit four five-inch cuttings from the diseased limb(s) including young woody tissue (pencil sized, previous season’s wood), leaves, and fruit stems (Figure 8).
Woody material should be from the previous season or older because it has been found that the current season’s growth can have variable pathogen concentrations. New research shows that leaves may test negative even though the wood they are attached to tests positive, therefore leaves by themselves are not recommended. Pencil sized wood from the previous season and fruit stems are the best tissue to sample.
Where to sample:
For trees with symptoms, sample from symptomatic limbs. For trees with no symptoms, sample from each leader. (Note that samples are only needed in unconfirmed blocks or adjoining trees.)
When to sample:
The week before harvest to mid-August.
Sample condition:
Keep tissue moist and cool (e.g., package with a cold pack). Old or dried tissue is more likely to have false negatives.
Download sampling fliers:
Scouting flier vf (2021.02.04)
Muestreo de X Fitoplasma y Little Cherry Virus flier WSU OSU (2021.02.04)
Controls
There is no cure, and an infected tree will remain infected for the rest of its life. There are no commercial products that have been shown to have an effect on the phytoplasma in scientific studies. Management requires a combination of strategies including planting pathogen free trees, identifying and removing infected trees, using cultural controls, managing vectors, and managing alternative hosts.
Pathogen-Free Planting Sources
Replacement trees must be obtained from pathogen-free planting stock. Nursery trees can be free of symptoms and still be infected. Use certified trees. Manage your risks—if in doubt, have the material tested before you buy or plant.
Identify and Remove Infected Trees
Primary control measures rely on identification and removal of infected trees. Remove infected trees following postharvest treatment for leafhoppers. Infected trees spread the pathogen to neighboring trees by insect vectors or via root-grafting from tree to tree. Treating stumps with herbicide immediately after cutting or injecting into trees before cutting trees (frill treatment) can help to identify adjoining root grafted trees and ensure roots are dead. Several glyphosate products are labeled; see these best management practices (BMPs) for tree removal. An early study showed that when infected trees were removed as soon as symptoms were observed, the disease incidence remained below two percent and decreased over time (Van Steenwyk et al. 1995).
Cultural Controls
Kaolin. Recent research has shown that kaolin clay (Surround) applications can reduce leafhopper vector populations in high pressure blocks by 48% percent and reduce leafhopper movement into the canopy (Marshall et al. 2024). Kaolin not only deters leafhoppers from orchards but it also repels leafhoppers from trees.
Extenday. Extenday can reduce populations in high- or low-pressure blocks by over 80% (Marshall et al. 2024). The effectiveness of Extenday is likely due to removing access to alternative and reproductive hosts in the drive row of the orchards, such as dandelion and other broadleaf weeds.
Grass in drive rows.Planting grass in drive rows can reduce peak leafhopper numbers by approximately 50% compared to weedy drive rows (Northfield, 2023, unpublished data). Leafhoppers cannot survive on grasses (Northfield and Cooper 2020). The most common plants fed on by the leafhopper vectors of X-disease phytoplasma are broadleaf weeds such as dandelion, mallow, lambsquarter, chickweed, knotweed, and plantain (Cooper et al. 2022).
Remove suckers. Root suckers provide easily accessible feeding sites for leafhopper vectors. Root suckers also tend to have higher levels of phytoplasma earlier in the season, likely due to movement of the pathogen from the roots where it overwinters (Wright et al. 2022). Removing root suckers may reduce movement of leafhopper vectors into the canopy and reduce transmission (Marshall, 2023, unpublished data).
Monitor and Manage Vectors
Consider timing. Both leafhopper population numbers and X-disease phytoplasma concentration in the tree are likely to be higher after harvest. When phytoplasma concentration in the tree is higher leafhoppers are more likely to acquire and transfer the pathogen. Concentrate monitoring and management efforts when risk is highest after harvest.
Monitor. Monitor leafhopper populations in the early and late season, including postharvest in order to manage populations not controlled by your general insect management program.
- Use yellow sticky cards or sweep nets (Purcell and Elkinton 1980).
- Hang sticky traps two to four feet from the orchard floor.
- Place traps on orchard borders, in areas of concern in your block, and throughout block. Use approximately one trap per two acres.
- Monitor every one to two weeks.
- Identify leafhoppers that vector X-disease phytoplasma (Figure 9).
- Use presence (an average of one vector leafhopper per trap) as a threshold to spray.
Rotate leafhopper products when populations are present. Manage vector leafhoppers when they are present based on monitoring. In Washington this is often after harvest. If leafhoppers are present, apply sprays and rotate between pesticide groups considering efficacy (Table 1). Due to the residual of common conventional products, sticky cards will likely show 21 to 30 days of control, necessitating four to six after-harvest sprays per season.
The following is an example spray rotation:
- group 3 pyrethroid (e.g. Warrior)
- group 4 neonicotinoid (e.g. Actara)
- a new active group
- back to a group 3 or group 4
- group 1 (e.g. Carbaryl) late in season when leaf-drop is not a concern.
Spray applications every two to three weeks should be the shortest interval needed. One reason more frequent sprays are not recommended is because it takes several weeks after feeding on an infected plant for a leafhopper to be able to transmit the phytoplasma. The phytoplasma must pass through the insect gut, into the “blood,” and to the salivary glands before it can be excreted into a new plant with the saliva. Additionally, with more frequent sprays, managers will likely exhaust the legally allowed number of applications before the end of the season when transmission is likely to be highest.
Manage alternative hosts of the phytoplasma and of the leafhoppers
Reducing broadleaf weeds in orchards can help reduce leafhopper numbers and potentially reduce transmission. Grasses are poor hosts for key vectors and are not a common host for phytoplasma. Apply broadleaf herbicides to the drive row as well as tree row. For example, in a recent study, herbicide applications reduced leafhopper vector numbers by up to 50%, likely by reducing the preferred habitat of the leafhoppers (Northfield, 2023, unpublished data). Healthy, weed-free grass strips compete with broadleaf weeds and supply a non-phytoplasma host environment.
Summary
Control of this disease requires a community-wide effort. What a neighbor does or does not do will affect adjacent orchards (and vice versa). The key to ending the current X-disease epidemic relies on reducing the amount of pathogen present in the state. This can only be done by removing infected trees, because it is from those trees that leafhoppers are acquiring the pathogen, then subsequently spreading it.
Available materials
Excerpt from the WSU Crop Protection Guide. For timings at which each pesticide can be used refer to the Crop Protection Guide.
Use pesticides with care. Apply them only to plants, animals, or sites listed on the labels. When mixing and applying pesticides, follow all label precautions to protect yourself and others around you. It is a violation of the law to disregard label directions. If pesticides are spilled on skin or clothing, remove clothing and wash skin thoroughly. Store pesticides in their original containers and keep them out of the reach of children, pets, and livestock.
YOU ARE REQUIRED BY LAW TO FOLLOW THE LABEL. It is a legal document. Always read the label before using any pesticide. You, the grower, are responsible for safe pesticide use. Trade (brand) names are provided for your reference only. No discrimination is intended, and other pesticides with the same active ingredient may be suitable. No endorsement is implied.
Additional Information
Internet resources
X-disease and Little Cherry Virus Scouting and Sampling Guide
Evaluations of Conventional and Organic Insecticides Against Leafhoppers: First Year Results
BMPs for tree removal for X-disease and Little Cherry Virus infected trees
Fruit Tree Planting Stock Certification Program
Removal Methods for X-Disease and Little Cherry Disease- Preliminary Trial Report
DuPont, S.T., Strohm, C., Molnar, C., Naranjo, R., Bishop, G., Case studies on tree removal for X-disease phytoplasma and Little cherry virus. Fruit Matters. August 8, 2020.
Harper, S., DuPont, T., Northfield, T. Alternative weedy hosts for X-disease phytoplasma. Fruit Matters March 2024. https://treefruit.wsu.edu/article/alternative-weedy-hosts-for-x-disease-phytoplasma/
Publications
Clarke, A.E., K.A. Catron, C. Reyes Corral, A.T. Marshall, C.G. Adams, W.R. Cooper, S.J. Harper, L.B. Nottingham, and T.D. Northfield. 2024. Colladonus spp. (Hemiptera: Cicadellidae) Vectors of X-Disease: Biology and Management in Western United States. Journal of Integrated Pest Management 15.
Harper, S.J., T.D. Northfield, L.B. Nottingham, et al. 2023. Recovery Plan for X-Disease in Stone Fruit Caused by ‘Candidatus Phytoplasma pruni’. Plant Health Progress 24: 258–295.
Northfield, T., and R. Cooper. 2020. Identifying Sources of X Disease in Cherry Orchards. Washington State Tree Fruit Research Commission Continuing Report, 2020.
Harper, S., A. Wright, and P. McCord. 2020. Understanding Little Cherry Disease Pathogenicity. Washington Tree Fruit Research Commission Continuing Report, 2020.
Nottingham, L., and T. Northfield. 2020. Insecticidal Control of Leafhoppers in Cherries. Washington State Tree Fruit Research Commission Continuing Report, 2020.
Videos
Scouting and Sampling for Little Cherry Disease
Symptoms of Little Cherry Virus and X-disease Phytoplasma. DuPont, S.T., Harper, S., Wright, A., Bishop, G. June, 2020.
Symptoms of X-disease Phytoplasma in Stone Fruit. Naranjo, R., Molnar, C., DuPont, S.T., Harper, S. Oct, 2020.
X-disease Vector Management Trials. Marshall, A., Northfield, T., Naranjo, R., DuPont, S.T. Aug, 2020.
X-disease Vector Management. Northfield, T., DuPont, S.T., Marshall, A., Naranjo, R. Aug, 2020.
Manejo de Vectores de Fitoplasma X (X-disease Vector Management). DuPont, S.T., Northfield, T., Naranjo, R. July 2020.
Síntomas de Fitoplasma X y Little Cherry Virus. DuPont, S.T., Harper, S., Wright, A., Bishop, G. June, 2020.
Síntomas de Fitoplasma X en Frutas de Hueso. Naranjo, R., Molnar, C., DuPont, S.T., Harper, S. Oct, 2020.
Contacts
Corina Serban, WSU Extension (509) 574-1595 corina.serban@wsu.edu
Scott Harper, Department of Plant Pathology, Washington State University (509) 786-9230 scott.harper@wsu.edu
Tobin Northfield, WSU Entomology (509) 293-8789 tnorthfield@wsu.edu
Tianna DuPont, WSU Extension (509) 293-8758 tianna.dupont@wsu.edu
Bernardita Sallato, WSU Extension (509) 439-8542 b.sallato@wsu.edu
Ashley Thomson, OSU Extension (541) 296-5494 Ashley.Thompson@oregonstate.edu
References
Beers, E.H. 1995. Effect of Rate and Timing of Biorational Materials for Control of First Generation White Apple Leafhopper Nymphs. Arthropod Management Tests 21(5).
Beers, E.H. 1996. Effect of Rate and Timing of Biorational Materials for Control of Second Generation White Apple Leafhopper Nymphs, 1995. Arthropod Management Tests 21(1): 6–7.
Chiykowski, L.N., and R.C. Sinha. 1982. Herbaceous Host Plants of Peach Eastern X-Disease Agent. Canadian Journal of Plant Pathology 4(1): 8–15. https://doi.org/10.1080/07060668209501330.
Cooper, W.R., A.T. Marshall, J. Foutz, et al. 2022. Directed Sequencing of Plant Specific DNA Identifies the Dietary History of Four Species of Auchenorrhyncha (Hemiptera). Annals of the Entomological Society of America 115: 275–284. DOI: 10.1093/aesa/saab053.
DuPont, S.T., E.H. Beers, A. Amiri, G.G. Grove, and L. Nottingham. 2020. Crop Protection Guide for Tree Fruits in Washington. Washington State University Extension Publication EB0419. Washington State University.
Fernandez, D.E., E.H. Beers, J.F. Brunner, M.E. Doerr, and J.E. Dunley. 2001. Mineral Oil Inhibition of White Apple Leafhopper (Homoptera: Cicadellidae) Oviposition. Journal of Entomological Science 36(3): 237–243.
Grant, J.A., and R.A. Van Steenwyk. 2000. Control of Mountain Leafhopper on Sweet Cherry, 1999. Arthropod Management Tests 25(1).
Harding, R.S., B.A. Nault, and A. Seaman. 2020. Potato Leafhopper Control in Snap Bean with Insecticides Allowed for Organic Production, 2019. Arthropod Management Tests 45(1).
Harper, S., A. Wright, and P. McCord. 2020. Understanding Little Cherry Disease Pathogenicity. Washington Tree Fruit Research Commission Continuing Report.
Hogmire, H.W., and T. Winfield. 1999. Insecticide and Acaracide Evaluation Against Leafhopper and Mite Pests 1999. Arthropod Management Tests.
Jensen, D.D. 1969. Comparative Transmission of Western X-Disease Virus by Colladonus montanus, C. geminatus, and a New Leafhopper Vector, Euscelidius variegatus. Journal of Economic Entomology 62(5): 1147–1150.
Jensen, D.D. 1971. Herbaceous Host Plants of Western X-Disease Agent. Phytopathology 61: 1465–1470.
Kuhar, T.P. 2009. Evaluation of Insecticide Treatments for the Control of Pests of Insects in Snap Beans. Arthropod Management Tests 34.
Laub, C.A., S. Tiwari, and R.R. Youngman. 2003. Efficacy of Foliar Insecticides Against Potato Leafhopper. Arthropod Management Tests.
Marshall, A.T. n.d. United State Department of Agriculture—Agricultural Research Service.
PAGE 11
Marshall, A. 2023, n.d. United States Department of Agriculture Agricultural Research Service.
Marshall, A.T., T.D. Melton, G. Bishop, et al. 2024. Cultural Control Methods Improve Management of Leafhopper Vector of X-Disease. Crop Protection 175.
Marshall, A.T., K.A. Catron, R.J. Orpet, R.T. Curtiss, T.D. Northfield, and L.B. Nottingham. 2025. Insecticide and Repellent Tests on Washington Leafhopper Vectors of Cherry X-Disease. Journal of Economic Entomology.
Molnar, C., S.T. DuPont, A. Thompson, and B. Sallato. 2022. Estimated Impact of X-Disease and Little Cherry Disease in Washington and Oregon from 2015 to 2020. Journal of Extension 40: 22.
Molnar, C., M.K. Shires, A.A. Wright, et al. 2024. Putting ‘X’ into Context: The Diversity of ‘Candidatus Phytoplasma pruni’ Strains Associated with the Induction of X-Disease. Plant Disease 108(9): 2677–2687.
Nielson, M. 1968. Biology of the Geminate Leafhopper, Colladonus geminatus, in Oregon. Annuals of Entomology Society of America 61(3): 598–610.
Northfield, T. Unpublished, 2023. Department of Entomology. Washington State University.
Northfield, T., and R. Cooper. 2020. Identifying Sources of X Disease in Cherry Orchards. Washington State Tree Fruit Research Commission Continuing Report.
Northfield, T., S.T. DuPont, S. Harper, and A. Marshall. 2023. X-Disease Vector Identification and Acquisition from Low Titer Trees. Final Report. Washington State Tree Fruit Research Commission.
Northfield, T., and L. Nottingham. 2020. Field Evaluation of Leafhopper Controls for X Disease Management. Washington State Tree Fruit Research Commission Continuing Report.
Northfield, T., and L. Nottingham. 2021. Field Evaluation of Leafhopper Controls for X-Disease Management. Washington State Tree Fruit Research Commission Final Report.
Nottingham, L., and T. Northfield. 2020. Insecticidal Control of Leafhoppers in Cherries. Washington State Tree Fruit Research Commission Continuing Report WTFRC Project: CH-20-103.
Nottingham, L., and T. Northfield. 2021. Insecticidal Control of Leafhoppers in Cherries. Washington State Tree Fruit Research Commission Final Report WTFRC Project: CH-20-103.
Patton, T.W., and G.P. Dively. 2002. Control of Potato Leafhopper Using Organic and Conventional Insecticides 2002. Arthropod Management Tests.
Purcell, A.H., and J.A. Elkinton. 1980. A Comparison of Sampling Methods for Leafhopper Vectors of X Disease in California Cherry Orchards. Journal of Economic Entomology 73: 854–860.
Reissig, H., D.H. Dunham, and C. Smith. 1995. Apple, Tests of Insecticides Against White Apple Leafhoppers, New York. Arthropod Management Tests 21: 45.
Severin, H.H.P., and E.C. Klostermeyer. 1950. Colladonus geminatus and C. montanus Life Histories on Virus-Infected and on Healthy Plants. Hilgardia 19: 553–560.
Shires, M.K., C. Molnar, S.J. Cowell, et al. 2024. Alternative Hosts of ‘Candidatus Phytoplasma pruni’ Identified Through Surveys and Vector Gut Content Analysis. Plant Health Progress.
Steenwyk, R.A.V., C.F. Fouche, J.A. Grant, and A.H. Purcell. 1993. Control of Mountain Leafhopper on Cherry, 1992. Insecticide and Acaricide Tests 18(1): 65–65.
Tubajikaa, K.M., E.L. Civerolob, G.J. Puterkac, J.M. Hashimd, and D.A. Luvisid. 2007. The Effects of Kaolin, Harpin, and Imidacloprid on Development of Pierce’s Disease in Grape. Crop Protection 26: 92–99.
Van Steenwyk, R.A., and C.F. Fouche. 1989. Control of Mountain Leafhopper on Cherry, 1988. Insecticide and Acaricide Tests 14(1): 60–61.
Van Steenwyk, R.A., C.F. Fouche, and D.M. Havens. 1988. Control of Mountain Leafhopper on Cherry, 1987. Insecticide and Acaricide Tests 13(1): 55–55.
Van Steenwyk, R.A., D.M. Havens, and R. Freeman. 1990. Evaluation of Trap Types for Two Vectors of Western X Disease: Colladonus montanus and Fieberiella florii (Homoptera: Cicadellidae). Journal of Economic Entomology 83(6): 2279–2283.
Van Steenwyk, R., B. Kirkpatrick, C. Fouche, J. Grant, and J. Uyemoto. 1995. Evaluation of an Abatement Program for Western X-Disease in Sweet Cherry. Plant Disease 79: 1025–1028.
Van Steenwyk, R.A., R.M. Nomoto, and J.A. Grant. 2002. Control of Mountain Leafhopper on Sweet Cherry, 2001. Arthropod Management Tests 27(1).
Van Steenwyk, R.A., S.K. Zolbrod, and R.M. Nomoto. 2003. Control of Mountain Leafhopper on Sweet Cherry, 2002. Arthropod Management Tests 28(1).
Wise, J.C., K. Schoenborn, and L.J. Gut. 2002. Season Long Broad Spectrum Insect Control. Arthropod Management Tests.
Wright, A.A., M.K. Shires, C. Beaver, G. Bishop, S.T. DuPont, R. Naranjo, and S. Harper. 2021. Effect of ‘Candidatus Phytoplasma pruni’ Infection on Sweet Cherry Fruit. Phytopathology 111(12): 2195–2202.
PAGE 12
Wright, A.A., M.K. Shires, C. Molnar, et al. 2022. Titer and Distribution of ‘Candidatus Phytoplasma pruni’ in Prunus avium. Phytopathology 112: 1406–1412.
treefruit.wsu.edu webpages may only be republished with prior author permission © Washington State University. Republished articles with permission must include: “Originally published by Washington State Tree Fruit Extension Fruit Matters at treefruit.wsu.edu” along with author(s) name, and a link to the original article