By Achour Amiri and Kutay Ozturk, WSU Plant Pathology. Updated April 2020. Printable version Blue_Mold (2020.04.15)
Blue mold is the most important postharvest disease of apples and pears worldwide and in the Pacific Northwest (Amiri and Ali, 2016; Rosenberger, 1990). Some strains of the blue mold causal fungus, Penicillium expansum, secrete patulin, a mycotoxin with mutagenic, neurotoxic, and gastrointestinal effects (Ramalingam et al., 2019). Therefore, blue mold is an economic concern not only to the fresh-fruit industry, but also to the fruit-processing industry since Penicillium contamination can result in patulin concentrations higher than the 50 µg/kg (ppb) limit in apple and pear processed products.
Casual Organism
Blue mold of apples and pears is thought to be mainly caused by Penicillium expansum, although several other Penicillium species such as P. solitum, P. commune, P. verrucosum, P. chrysogenum and P. regulosum, have also been reported to cause blue mold decay. Although the growth of Penicillium spp. is limited in cold storage, the fungus can grow at temperatures as low as -3ºC (27ºF) and germination can occur at 0ºC (32ºF) (Rosenberger and Xiao, 2014).
Symptoms
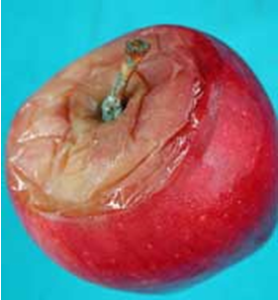
Blue mold originates primarily from wounds, such as punctures, splits, bruises and limb frictions, infected by Penicillium spp. At early infection stages, blue mold symptoms include light tan to dark brown circular lesions with very sharp margin between diseased and healthy tissues (Fig 1A-C). The decayed tissue is medium-soft and watery. Decayed tissue can be readily separated from the healthy tissue, leaving a “bowl-like” cavity (Fig. 1D). Green spore masses may appear on the decayed area, starting at the infection site. As the decayed area ages, green spores may turn blue giving the common name of “blue mold” to the disease. Decayed fruit has an earthy, musty odor. Blue-green spore masses (Fig. 1G) on the lesion and the associated musty odor are diagnostic of blue mold. When decay has fully developed and the lesion softens, green-blue spore masses may be less apparent and blue mold can be misdiagnosed as Mucor rot. However, Mucor rot is characterized by a sweet odor and usually softens much quicker than blue mold (Table 1). Blue mold can also originate from infections through the stem bowl (Fig. 1E), where potential openings may have been caused by stem removal or through cracks and splits that are common on cultivars such as Gala and Honeycrisp. Calyx-end blue mold (Fig. 1F,I) may occur as a result of fruit drenching or in some cases due to core rot spreading to the surface of the fruit.
Figure 1. Symptoms and signs of blue mold caused by Penicillium spp., mainly P. expansum) on apples and pears. Photo credit: Achour Amiri (WSU-TFREC) and Chang-Lin Xiao (USDA-ARS).
Table 1. Disease comparison between blue and Mucor rot
| Trait | Blue mold | Mucor rot |
|---|---|---|
| Texture | soft, watery | very soft, juicy |
| Color of decayed area | light tan to dark brown | light brown to brown |
| Signs of pathogen | white mycelium, blue or blue-green spore masses | gray mycelium with dark sporangia |
| Color of internal flesh | brown | light brown to brown |
| Odor | earthy, musty | sweet |
Infection and Disease Cycle
Penicillium spp. are typical postharvest pathogens, and although some inoculum can be found in orchard soils and on organic debris on the orchard floor, fruit are seldom infected by Penicillium while on the tree. Penicillium survive easily as airborne spores in storage room air, walls, floors and bins. When bins are immersed in dump-tanks, large amounts of spores can be released into the water and carried out in flume water infecting additional fruit on the packing line. Infections may spread through extended storage, especially for packers who pre-size and store wet fruit (Amiri and Bompeix, 2005; Sanderson and Spotts, 1995). Infection by P. expansum may start immediately after harvest on fresh wounds caused during picking and handling (Amiri and Bompeix, 2005). These lesions will develop slowly in cold storage and may produce large amounts of spores that are air-disseminated by continuous fan movements during storage to infect additional fruit, air, walls and bins. Infections by Penicillium occurring through lenticels are rare but possible, especially as the fruit over-mature and lenticels may breakdown. On rare occasions, remaining infected parts of flowers can serve as a source of inoculum of Penicillium and other pathogens to infect seeds inside the fruit that may cause “core rot” later in storage. Although certain cultivars such as Honeycrisp and Gala may seem more susceptible to blue mold infections, all current commercial apple and pear cultivars are susceptible to blue mold, especially through wounds.
Cultural Controls
Orchard sanitation to remove decayed fruit and organic debris on the orchard floor helps reduce inoculum levels of Penicillium spp. in the orchard. Good harvest and handling practices designed to minimize fruit punctures and bruises are critical to minimize blue mold infections.
However, because postharvest environments create more favorable conditions for infections and decay development, mainly due to high humidity levels, postharvest sanitation practices have a greater impact on blue mold control. It is important to clean and sanitize rooms (air, walls, floors) and bins at least once a season before using them to store a new crop. Sanitation of the packing-line from the dump-tank all-the-way to the sorters needs to be done regularly during the packing season. Several sanitizers, i.e. chlorine and chlorine dioxide, hydrogen peroxide, organic aids, electrolyzed water and ozone, are available and all have different efficacies and uses (Bernat et al., 2018; Feliziani et al., 2016). It is recommended to consult extension specialists or consultants in your area and the product labels to optimize sanitation. Recent cold or hot fogging technologies have shown good levels of efficacy in sanitizing larger facilities (entire rooms, lines, stacked bins) more efficiently.
Chemical Controls
Most current preharvest fungicides applied days before harvest may not provide the highest level of efficacy as Penicillium infections typically occur after harvest. Therefore, postharvest fungicide applications remain the most effective chemical way to control blue mold.
Currently, there are four single-site postharvest fungicides labeled for postharvest application (Table 2). Thiabendazole (Mertect) has been largely used in the PNW since the 1970s until early 2000 when two new postharvest fungicides, fludioxonil (Scholar) and pyrimethanil (Penbotec), were registered for postharvest decay management. In 2016, difenoconazole was labeled for postharvest application in pome fruit and other commodities.
With current absence of resistant strains these four fungicides have a high level of efficacy against blue mold, with fludioxonil shown to have the highest efficacy. For decades, postharvest fungicides have been applied as a drench at harvest or as a spray on the packing-line. However, in recent years, thermonebulization (also called fog or aerosol or dry application) has become common in the PNW pome fruit industry. One of the advantages of fog application is that it reduces the spread of spores between bins compared to when non-sanitized bins are used for drenching. Although fog application still requires more optimization for other diseases, it has been shown to have a better efficacy against blue mold compared to drenching. Formulations to apply thiabendazole, pyrimethanil , and fludioxonil through TNB exist already, whereas difenoconazole’s dry formulation is pending. Besides these four single-site fungicides, captan, a multi-site fungicide, is also registered for postharvest application. Its efficacy is fair and may help for short-term storage and for managing fungicide resistance development. Postharvest fungicides should be applied soon after harvest because the more time that elapses after harvest, the higher the risk for decay to develop in storage.
Table 2. List and efficacy of postharvest fungicides registered to control blue mold and other decay rots
| Active Ingredient | Trade names | FRAC group | Rate | Available for application via Drench | Available for application via TNB* | Efficacy against Blue Mold | Resistance reported in WA | Resistance reported in PNW | Resistance reported elsewhere |
|---|---|---|---|---|---|---|---|---|---|
| Thiabendazole | TBZ, Mertect, eFog 100 |
1 | see label | Yes | Yes | Very good | ++** | + | ++ |
| Pyrimethranil | Penbotec, eFog 160 |
9 | see label | Yes | Yes | Very good | + | + | + |
| Fludioxanil | Scholar, eFog 80, Actimist | 12 | see label | Yes | Yes | Excellent | Tolerance☨ | Tolerance | + |
| Difenoconazole + fludioxonil |
Academy | 3 + 12 | see label | Yes | Pending | Excellent | – | – | – |
| Captan | Captec 4L | M04 | see label | Yes | Yes | Fair | NA | NA | NA |
* TNB = thermonebulization (fog);
** – = resistance not reported, + = resistance has emerged, ++ = some resistance reported;
☨ Isolates with tolerance or reduced sensitivity.
Fungicide Resistance Management
Penicillium expansum is considered to have a medium to high risk for fungicide resistance development. Because the fungus sporulates profusely, there is a risk that many spores will be exposed to the applied fungicide which increases the risk of selecting for resistance. Resistance to thiabendazole has been reported in the PNW (Li and Xiao 2008) and elsewhere. In Washington State, frequencies vary between packers based on the usage frequency. Resistance to pyrimethanil (Penbotec) has been found to range between 0 and 50% in Washington (Caiazzo et al., 2014, Amiri and Pandit, 2019) whereas 5% to 10% of the population showed tolerance to fludioxonil (Scholar) (Amiri and Pandit 2019). Given the known risk and the limited number of postharvest fungicides available, it is critical that these materials are used appropriately to limit the development of fungicide resistance. Knowing what type and level of resistance in each warehouse is the first step in developing an effective spray program. WSU researchers (Contact Dr. Amiri) can help with conducting a risk assessment. The second step is to implement sound annual cleaning and sanitation to remove or reduce the residual inoculum from previous seasons. It is critical to apply a different FRAC group for postharvest treatments each season. Although resistance to thiabendazole has been documented, 50% of PNW packers can still use this fungicide if they follow good sanitation practices. It is recommended not to apply thiabendazole (FRAC 1) postharvest if Topsin-M (FRAC1) was used preharvest. The two most effective fungicides pyrimethanil (FRAC 9) and fludioxonil (FRAC 12) can be alternated on a yearly basis. In some cultivars, known to be susceptible to bull’s eye rot (BER), is recommended to apply FDL with thiabendazole to improve control of BER. Academy (DIF + fludioxonil) has shown high efficacy levels against blue mold after seven months of cold storage (Ali and Amiri 2018; Jurick et al. 2018). In Washington State, Academy can be applied via drench at harvest given that packers follow specific fungicide waste management practices (https://agr.wa.gov/wastepesticide). A formulation for a dry application of Academy is under development. For other states and regions, please contact your local agencies for additional information. For organic packers, there are not many options to fight blue mold postharvest. Sanitation is more critical when growing, storing and packing organic fruit. A recent study has shown that a combination of peroxy-acetic acid and hydrogen peroxide applied at 50 fl oz/100 gal via dip was effective in controlling several postharvest diseases.
Use pesticides with care. Apply them only to plants, animals, or sites listed on the labels. When mixing and applying pesticides, follow all label precautions to protect yourself and others around you. It is a violation of the law to disregard label directions. If pesticides are spilled on skin or clothing, remove clothing and wash skin thoroughly. Store pesticides in their original containers and keep them out of the reach of children, pets, and livestock.
YOU ARE REQUIRED BY LAW TO FOLLOW THE LABEL. It is a legal document. Always read the label before using any pesticide. You, the grower, are responsible for safe pesticide use. Trade (brand) names are provided for your reference only. No discrimination is intended, and other pesticides with the same active ingredient may be suitable. No endorsement is implied.
References
Amiri A., and Pandit LK. 2019. Fungicide resistance in Penicillium expansum from pome fruit in the U.S. Pacific Northwest. Phytopathology 109-10: S2.75.
Ali EM., and Amiri A. 2018. Selection pressure pathways and mechanisms of resistance to the demethylation inhibitor-difenoconazole in Penicillium expansum. Frontiers in Microbiology 9:2472.
Amiri A., and Ali. MD.E. 2016. Prevalence of storage decays of apple: Lessons from the 2016 statewide survey. https://treefruit.wsu.edu/news/prevalence-of-storage-decays-of-apple-lessons-from-the-2016-statewide-survey/
Amiri A., and Bompeix G. 2005. Diversity and population dynamics of Penicillium spp. on apple in pre-and postharvest environments: consequences for decay development. Plant Pathology 54:74-81.
Bernat M., Casals C., Teixido N., Torres R., and Usall J. 2018. Efficacy of environmental-friendly disinfectants against the major postharvest pathogens of stone fruits on plastic and wood surfaces. Food Science and Technology International 25:109-119.
Caiazzo R., Kim YK., and Xiao CL. 2014. Occurrence and phenotype of pyrimethanil resistance in Penicillium expansum from apple in Washington State. Plant Disease 98:924-928.
Feliziani E., Lichter A., Smilanick JL., and Ippolito A. 2016. Disinfecting agents for controlling fruit and vegetable diseases after harvest. Postharvest Biology and Technology 122:53-69.
Jurick, MW., Macarisin, O., Gaskins Vl., et al. 2018. Baseline sensitivity of Penicillium spp. to difenoconazole. Plant Disease 103:331-337.
Li, HX., and Xiao CL. 2008. Baseline sensitivity to fludioxonil and pyrimethanil in Penicillium expansum populations for apple in Washington State. Postharvest Biology and Technology47:239-245.
Ramalingam, S., Bahuguna, A., and Kim, M. (2019). The effects of mycotoxin patulin on cells and cellular components. Trends in Food Science and Technology, Vol. 83, pp. 99–113.
Rosenberger and Xiao, 2014. Postharvest Diseases. pages 75-86 in Compendium of apple and pear diseases and Pests. Second Edition, American Phytopathological Society Press. St. Paul. MN.
Rosenberger, DA. 1990. Blue mold. Pages 54-55 in: Compendium of Apple and Pear Diseases. A. L. Jones and H. S. Aldwinckle (ed.)., American Phytopathological Society Press St. Paul, MN.
Sanderson PG., and Spotts RA. 1995. Postharvest decay of winter pear and apple fruit caused by species of Penicillium. Phytopathology 85:103-110.
Contact
 Dr. Achour Amiri,
Dr. Achour Amiri,
Postharvest Pathology
Tree Fruit Research and Extension Center
Wenatchee, WA
a.amiri@wsu.edu
509-293-8752
treefruit.wsu.edu articles may only be republished with prior author permission © Washington State University. Reprint articles with permission must include: Originally published by Washington State Tree Fruit Extension at treefruit.wsu.edu and a link to the original article.