Twospotted spider mite
by Elizabeth H. Beers and Stanley C. Hoyt, originally published 1993
Tetranychus urticae Koch (Acari: Tetranychidae)
Hosts
Its innumerable hosts include many weeds, field crops, ornamental and house plants, vegetables, forage crops, small fruits and tree fruits. Among the tree fruits, apple, pear, peach, nectarine, apricot, cherry (sweet and sour), plum, and prune are suitable hosts. High mite populations encountered on pear are likely to be either twospotted or McDaniel spider mite. Twospotted spider mite used to be rare on apple, but has become more common in recent years, greatly surpassing McDaniel spider mite as a problem.
Life stages
Egg

The egg is spherical and about 1/150 inch (0.14 mm) in diameter. When first deposited, the egg is translucent, taking on the greenish tinge of the leaf where it is laid. It becomes more opaque as it matures, finally turning a pale yellow. The red eyespots of the embryo are visible just prior to hatch.
Immatures
The larva is round, about the same size as the egg, and has three pairs of legs. Initially it also is translucent (except the red eyespots), but once it begins feeding, it turns pale green to straw color, and the characteristic black spots begin to form on the dorsum (back). The protonymph is larger and more oval, and has four pairs of legs, as do all succeeding stages. The two dorsal spots are more pronounced, and the green color is slightly deeper. The deutonymph is slightly larger than the preceding stage, and males can be distinguished from females at this stage by the smaller size and more pointed abdomen. Each immature stage goes through three phases: active feeding, a quiescent period, and a molt. The integument (the outer covering of the body) may take on a silvery appearance in the quiescent stage as it separates from the skin below in preparation for the molt.
Adult

The adult male is smaller than the female and is characterized by its distinctly pointed abdomen. It sometimes has an orange or brown tinge and is more active than the female. The female is about 1/60 inch (0.42 mm) and more robust than the male and is more oval in shape. Color of the female can also vary. Typically, it is a pale leaf-green, but it can be tinged with yellow, brown and orange. As the name implies, there are generally two distinct spots on the front half of the dorsum behind the eyes. These spots are caused by pigments in the digestive tract which is why the size, distinctness, and pattern of spots can vary considerably among individuals or at different times during the life span of a single individual. Overwintering females are usually a distinct solid orange, and the spots disappear. During this stage, they can only be distinguished from McDaniel mite by slide mounting and examining the integument of the dorsum for a diamond-shaped section of striations.
Life History
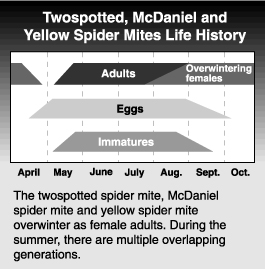
The twospotted spider mite overwinters as orange colored adult females in the duff at the base of trees and in sheltered sites beneath bark scales. Only females are known to overwinter. They emerge from their overwintering sites about the half-inch green stage of apple development. As these mites begin feeding, they gradually lose their orange color and gain their normal greenish hue and dorsal spots. After about 2 to 5 days, egg laying begins, primarily on the underside of newly expanded leaves, and later on the fruitlets. Overwintering females lay an average of 39 eggs over a life span of 23 days, considerably fewer than the summer forms. These eggs may take up to 3 weeks to hatch, depending on temperature. From this point onwards, generations begin to overlap. Summer-form females can lay about 100 eggs over a period of 30 days. Egg hatch takes only one or two days during the warm part of the summer, and the entire generation time (oviposition to adult) may take only 10 days. When leaf quality begins to decline (e.g., from excessive mite feeding), or when cooler temperatures and shorter day lengths occur during the fall, the orange overwintering forms are again produced.
Damage, Biological Control, and Management
see sections under European red mite
Monitoring
Twospotted Spider Mite Gallery
McDaniel spider mite
by Stanley C. Hoyt and Elizabeth H. Beers, originally published 1993
Tetranychus mcdanieli McGregor (Acari: Tetranychidae)

The McDaniel spider mite was first described on raspberry in Michigan in 1930 but has gained pest status only on tree fruits in the Pacific Northwest, Utah and certain areas of California, usually in the drier areas. It has been most studied as a pest of apples in central Washington where it has been a pest since the 1930s. Widespread outbreaks occurred during the late 1950s and early 1960s as a result of severe disturbance of biological control. These outbreaks led to the orignal studies on integrated mite control, which has been successfully implemented in the majority of Washington apple orchards since that time. While McDaniel mite was the primary outbreak species in central Washington during that period, it has become relatively uncommon in recent years.
Hosts
Although McDaniel spider mite is considerably less polyphagous than twospotted spider mite, its host range is still fairly broad. It attacks most deciduous tree fruits (apple, pear, sweet and sour cherry, prune, peach and apricot), some field and vegetable crops (squash, asparagus, alfalfa, clover), and a number of weeds (mallow, milkweed, knotweed, ragweed, mustard, dock, wild buckwheat, wild lettuce). In a study of numbers and survival of eggs, Red Delicious apple, sweet cherry and apricot were better hosts for McDaniel spider mites than pear, peach and sour cherry.
Life stages
Egg

The egg is spherical and about 1/150 inch (0.13 mm) in diameter. It is translucent when first laid but becomes darker. Just before hatch it assumes a dull ivory color, and the red eyespots of the embryo are visible. McDaniel spider mite eggs cannot be distinguished from twospotted spider mite eggs.
Immatures
The larva has three pairs of legs and is about the same size as the egg. It has little or no color until it begins feeding when it takes on a greenish tinge from leaf chlorophyll. The quiescent stage is about 1/12 inch (0.20 mm) long. The protonymph is oval and dark green and has four pairs of legs. The female is 1/100 inch (0.25 mm) long, and the males are slightly smaller. The deutonymph also has four pairs of legs and is generally the same color and shape as the preceding stage, only larger. The female, which is about 1/70 inch (0.35mm) long, can readily be distinguished from the male at this stage. The male has characteristic slender, tapered abdomen and is distinctly smaller than the female.

Adults
The adult female is about 1/60 inch (0.44 mm) long, whereas the male is only about 1/80 inch (0.29) long. The adult female, like the deutonymphs, can also be distinguished from the male by its shape. The female has a broad oval shape, whereas the male has a slender, more pointed abdomen. The dark spots on the abdomen that characterize this species occur on all stages but are most distinctive in the older stages. The earlier stages may be difficult to distinguish from twospotted spider mite because of the variability in both species. McDaniel spider mite has multiple pairs of spots, some of which always occur in the posterior portion of the abdomen.
Twospotted spider mite, on the other hand, usually has two fairly distinct spots, and the area of pigmentation is confined to the front half of the abdomen. Both species lose their spots during a molt and do not regain them until they begin feeding again. Because of the variability, identification may be difficult. However, slide mounting and examination under high magnification make it possible to distinguish the two species.
Life history
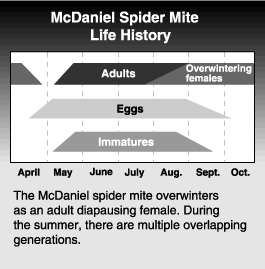
The McDaniel spider mite overwinters as an orange-colored diapausing female under bark scales or in litter at the base of the tree. Overwintering mites, which are often in large masses, produce extensive webbing. The mites emerge from their overwintering sites in March or April, depending on the area, but at about the time fruit buds begin to open. They move to the green tissue and begin to feed. Mites may move directly into the tree fruit host or, if available, to nearby weed hosts. As they feed, they lose their orange color and take on the normal greenish tinge and characteristic dorsal spots. After a few days of feeding, egg laying begins.
Eggs are initially laid on the undersides of leaves, but when populations are high both sides of the leaves are colonized. In apple, the mites tend to stay close to the main trunk and scaffold limbs initially so their distribution in the early part of the season covers a cone-shaped area in the center of the tree. Although there is some lateral distribution, McDaniel spider mites do not tend to infest terminal growth because they prefer older foliage.

Eggs are laid either in or beneath the webbing, possibly providing protection from some chemical sprays and predators other than T. occidentalis. Summerform females can lay up to 150 eggs over a 5- to 6-week period. The time required to complete development at very high temperatures can be as little as 6 days, but at typical midsummer field temperatures development requires about 8 to 11 days. There are up to 10 overlapping generations per year in central Washington, depending on the temperatures. In the absence of predators, populations peak in late July to mid-August, then decline. Overwintering forms may be produced as early as May if foliage quality deteriorates, but they are usually produced from mid-July through September. The males die with the onset of cold weather, and the orange-colored females seek sheltered sites to spend the winter.
Damage
Spider mites all cause the same type of feeding damage (see damage section under European red mite). In addition, the McDaniel spider mite forms very dense webs on leaves and fruit. Webs on the leaves may prevent good spray penetration, making chemical control more difficult. Mites and webbing in the calyx end of fruit have also been noted as a problem since this species is regulated by quarantine in certain countries.
Monitoring
The basic scheme for monitoring spider mites is the same for all three of the more common species. See the general description under European red mite and the sampling plan for McDaniel and twospotted mites below.
McDaniel Spider Mite Gallery
Yellow spider mite
by Elizabeth H. Beers and Peter H. Westigard, originally published 1993
Eotetranychus carpini borealis (Ewing) (Acari: Tetranychidae)
The yellow spider mite is an occasional pest in the Rogue River Valley of Oregon. It has never gained pest status outside this area, although it occurs throughout the northwestern United States and southwestern Canada.
Yellow Spider Mite Gallery
European red mite
by Elizabeth H. Beers and Stanley C. Hoyt, originally published 1993; revised December 2007
Panonychus ulmi (Koch) (Acari: Tetranychidae)
The European red mite, as the name implies, was originally brought to the United States from Europe, where it is widely distributed and has been a prominent pest for many decades. In the United States, it was first recorded in Oregon in 1911 and has since become common throughout the United States and Canada. European red mite has a long history of developing resistance to miticides. As a result, there has been much work on biological control, with many instances of success. Outbreaks of European red mite do not usually occur in unsprayed habitats. Only release from biological control agents (through the use of pesticides) allows this pest to build to the levels seen in commercial orchards.
Although McDaniel spider mite was the outbreak mite species during the 1960s, European red mite has become the most common mite pest of apple in recent years. Twospotted spider mite is generally the most typical species on pear, but European red mite has also been noted more frequently on this crop.
Hosts
European red mite is a pest of many crops and ornamentals. Of the tree fruits, it is most commonly a problem on apple, pear, plum, prune and cherry, although mite problems on pear are more likely to be twospotted or McDaniel spider mite. Other hosts include peach, almond, grape, raspberry, currants, gooseberry, rose, black locust, elm, hawthorn, privet, lilac, chestnut, and alder buckthorn (Frangula). Although it will attack all apple varieties, it tends to build up higher populations on Red Delicious and Rome. Golden Delicious trees rarely support as high a population as do Red Delicious trees in the same orchard. Other cultivars reported as less susceptible to attack are McIntosh and Winesap. Pear cultivars also vary in their susceptibility to mite feeding.
Life stages
Eggs
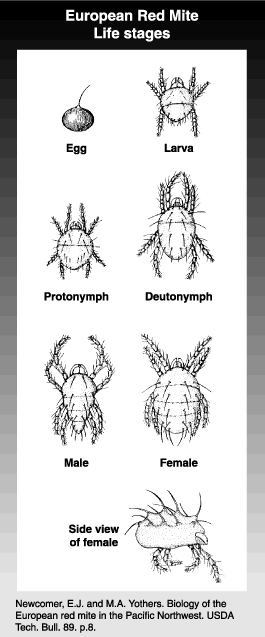
Overwintering eggs are found on twigs and small limbs, especially in the crevices, beginning in the late summer or fall, throughout the winter, and up until they hatch in spring. They are about 1/160 inch (0.15 mm) in diameter, are brick red and have a stipe, or stalk. Summer eggs are laid on the foliage and, if populations are high, on the fruit, especially the calyx end. These are slightly smaller and about the same color as the overwintering eggs. After the egg hatches, the empty shell is transparent.
Immatures
There are three immature forms (the larva, protonymph and deutonymph). The larva is only slightly larger than the egg and is an orange-red. It can be distinguished from other immature stages by having only three pairs of legs. The protonymph and deutonymph are successively larger and have four pairs of legs, as do adults. Immature stages of European red mite are reddish in color but sometimes have a greenish cast. Between each stage is a quiescent stage which precedes a molt. The integument can appear silvery during this stage as it separates from the layer beneath.
Adult
The adult female is about 1/72 inch (0.35 mm) in length, typically a solid brick red, and is characterized by her oval shape and strong white bristles on the back of the abdomen. The bristles, which have white bases, look like white spots on the back. The male is a yellowish red tinged with red. The male is only about 1/80 inch (0.30 mm) long and is more slender than the female. It has a tapered abdomen.
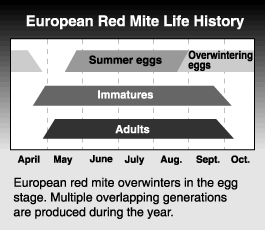
Life history
The European red mite passes the winter in the egg stage. Eggs are usually laid on the lower sides of small branches and twigs. They are often found around the forks of two branches, in crevices and other rough areas. Egg hatch begins at about the tight cluster to pink stage of Red Delicious development and is largely complete within in 7 to 10 days.
The larvae move to young leaves to feed. Immature mites often rest and feed more extensively on the bottom side of the leaves, but adult mites inhabit both surfaces. Although mites feed on newly expanded leaves early in the season, they are more likely to be found on hardened-off foliage when it is available.
The sex of the mite can be determined at the deutonymphal stage when females are already larger and more oval than the males, but the difference does not become obvious until the adult stage. The first adults appear around petal fall on apple. Males emerge first. The male waits near a quiescent female until she emerges and mates with her immediately. After about two days, females begin to lay eggs. A female mite lives 15 to 20 days and lays 30 to 35 eggs on average. Eggs from unfertilized females produce only males, by arrhenotokous parthenogenesis, but eggs from fertilized females produce both males and females. Eggs are deposited on both sides of the leaf but are concentrated near veins and midribs. In hot weather, they can complete their life cycle in as little as 10 days, though it can take up to 25 days in cooler weather. There are 6 to 8 generations per year, which overlap more as the season progresses. Populations usually peak in late July or August, although the peak is probably modified by pesticide use patterns.

When mite populations reach high levels, they can disperse by a process known as “ballooning.” Mites, sometimes in great masses, crawl to a high point or a tip of a branch and raise the anterior part of the body. The mite spins a silken thread, which is caught by air currents, taking the mite along with it. This is one of the primary means of mite dispersal to nearby trees where canopies do not overlap.
Females start laying overwintering eggs in mid-August to September. An over-exploited food resource (i.e., badly bronzed foliage) can lead to earlier deposition of overwintering eggs. Females usually lay eggs on the bark, but when populations are high they sometimes deposit them in the calyx end of the fruit. Although European red mite does spin webbing, it does not do so to the same extent as do twospotted or McDaniel spider mite.
^top
European Red Mite Gallery
Damage
Note: The following sections on damage and management cover all four spider mite species. As far as it is known, all of these species feed in the same manner, and no attempt has been made to differentiate the effects of one species vs. another. In the Pacific Northwest, economic injury research on apple was done primarily with McDaniel spider mite, and research on pear was done with twospotted spider mite. Where populations are mixed, there is no reason to assume that feeding by one species differs in its effect on the tree than feeding by the others. Rust mite damage is different, however, and is treated in the appropriate section.

Mites feed by inserting their mouth parts into leaf cells to suck out the contents, including the green pigment chlorophyll. The individual spots initially look white, giving the leaves a stippled appearance. As damage progresses, the infested leaves take on a brown hue, commonly called bronzing. Tree species, and cultivars within species, differ substantially in their reaction to mite feeding. Although Red Delicious trees tend to build higher populations of mites, Golden Delicious is more susceptible to damage by mites. Apples, in general, are more tolerant than pears. Whereas damage to apple foliage will cause bronzing and eventually some premature leaf abscission in August, damage to pear leaves can lead to “transpiration burn,” where leaves develop large necrotic areas and are shed. This can occur in midseason or whenever substantial mite damage is combined with some additional types of stress on the trees.
Of the common Pacific Northwest pear varieties, the ranking from most to least susceptible to damage is Anjou, Bosc, Bartlett, and Comice. The red strains of pear cultivars are not prone to transpiration burn or defoliation and are considered tolerant of mite damage. As well as damaging foliage, spider mites feed on the epidermis of pear fruit, causing russeting. High populations late in the season are most likely to cause fruit russet.
Prune and other stone fruits have an intermediate reaction. Although the leaves bronze, as opposed to developing transpiration burn, defoliation is more common than on apple.

The effects of mite damage to foliage have been much studied but are still poorly understood. On apple, studies have examined photosynthesis, transpiration, shoot growth, trunk and limb growth, root growth, fruit size and rate of growth, fruit color (internal and external), soluble solids, firmness and other quality parameters, return bloom and return fruit set. At best, it can be stated that under some circumstances, mite damage affects one or more of these parameters. Similar studies on pears indicate a marked reduction in fruit size and return crop.
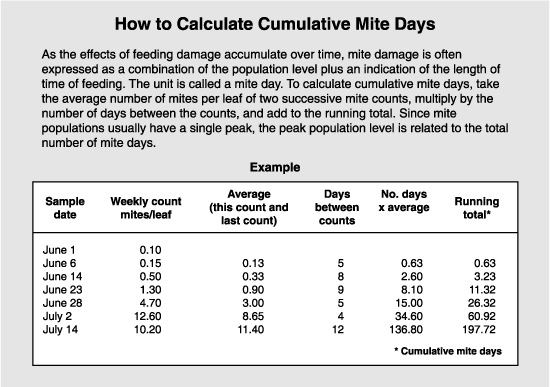
As the effects of feeding damage are cumulative over time, mite damage is often expressed as a combination of the population level (intensity) plus an indication of length of time of feeding (duration). The unit is called a mite day, or the damage caused by one mite feeding for one day. Thus, 100 mite days could be the product of 100 mites feeding for one day, or 10 mites for 10 days.
Since mite population curves usually have a single peak, the peak population level is related to the total number of mite days. Some studies have used peak populations as a measure of the economic injury level. The variability in damage makes it difficult to set an economic injury level or an treatment threshold that will be valid for all cultivars, times of season, weather, crop load, etc. For apple, a somewhat conservative level of 800 mite days was set for Washington conditions. This corresponds with a peak population of 30 mites per leaf. On pears, the greater susceptibility is reflected in a lower threshold. The economic injury level is set at 5 mites per leaf, and the treatment threshold used to prevent that from occurring ranges from 0.5 to 2 mites per leaf.
Monitoring
Mites are difficult to detect initially due to their small size, but the effects of substantial feeding will be evident without getting out of the car. Mites are visible on foliage, but most people will need a hand lens to distinguish the species. Reddish stains on the fingers after handling infested leaves are usually diagnostic for European red mite.
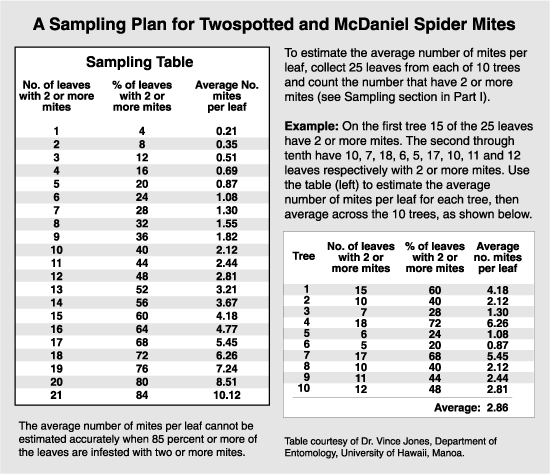
There are several approaches to monitoring mite populations. One is an in situ examination. Take a hand lens and examine 10 leaves from each of 10 trees per block (total of 100 leaves), and count the number of mites. This is laborious and subject to counting error if populations are high. However, if other equipment is lacking, it can give a reasonable assessment of mite populations.
A standard approach is to collect leaves in the field and bring them back to where a leaf brushing machine and a microscope can be used. The leaf brushing machine is a device with opposable rollers covered with soft bristles which brush mites from the leaf surface down to a revolving glass plate coated with a slightly sticky substance (e.g., liquid detergent). The mites are immobilized on the surface of the plate, and the microscope aids in counting individual species and stages. Usually a paper grid with 20 wedges marked on it is placed beneath the glass plate to help keep track during counting. A grid that can be printed for use is available. The total mites on the plate divided by the number of leaves brushed onto that plate yields the estimate of mites per leaf. When populations are high, only a fraction of the plate is counted (one-half or one-quarter). This method is also laborious and subject to error but is probably the most accurate of the methods presented. It has been the standard method on which much of the economic injury evaluations have been made.
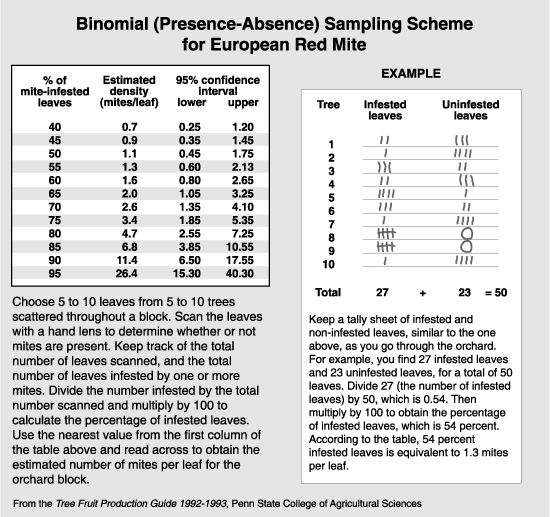
A technique called binomial sampling has become more popular recently because it simplifies the estimating procedure with the use of statistics (see the article on Sampling). The binomial sample can be done in the field, and each leaf examined is rated as to the presence or absence of mites. The percentage of leaves infested (i.e., with one or more mites) is statistically related to the number of mites per leaf, and can be looked up on a table or chart made expressly for that species. The primary drawback to binomial sampling in the arid regions of the West is that the number of mites per leaf cannot accurately be estimated when 85% or more of the leaves are infested. This means that the highest level that can be estimated with accuracy is 10 mites per leaf for the twospotted and McDaniel spider mite, which is well below the treatment threshold of 30 mites per leaf. The highest level that can be accurately predicted with the European red mite is 26 mites per leaf. An additional drawback is that rust mites and T. occidentalis, which are important components in decision making, cannot be evaluated in the binomial sample. However, this method may be used as an indication if further sampling is necessary. A binomial sample indicating that 95% or more of the leaves are infested with either European red mite or the Tetranychus species shows the need for a standard brushing machine sample.
Because mites are on the tree from the pre-bloom period through fall, it is important to make repeated assessments for mites. You cannot take a single sample at a critical point in its life cycle and be finished for the season. Mites can build up at different times in different blocks or years. The only time that might be termed a single critical point is for the overwintering eggs. However, delayed-dormant oil applications are made routinely in the Pacific Northwest so this is rarely considered a decision making point. Where biological control occurs, many people do not monitor populations. It is advisable to sample at least once in mid- to late June to insure that rust mites and predators are present in adequate numbers.
Biological control
The predatory phytoseiid mites are the most important biological control agents in the Pacific Northwest. Typhlodromus occidentalis is by far the most important predator in most areas, although in some of the more humid areas of Oregon, Typhlodromus pyri can also play a role. A stigmaeid mite, Zetzellia mali, is often found in low numbers in orchard blocks but usually provides only supplemental control. Stethorus picipes, a small black coccinellid (ladybird) beetle, can consume large numbers of mites but is not attracted to the orchard until the infestation level is quite high. Campylomma (a mirid true bug) is also reported to be an effective mite predator in Europe, but its predatory role in the Pacific Northwest has not been explored. Furthermore, its habit of feeding on fruit early in the season outweighs its benefit as a predator. Early stages of Deraeocoris bugs and lacewings also feed on mites.
Management
Rust mites play an important role in integrated mite management as an alternate food source for predatory mites. This is a critical component in the system, since it allows predators to maintain themselves during periods of low spider mite densities. If predators are in low to moderate numbers, spider mite populations may not build up much past the level of detection, and consequently will stay well below the treatment threshold. Rust mite populations tend to crash during periods of prolonged hot weather; they are usually most abundant during spring and early summer and then again in fall. In the hottest areas of the Pacific Northwest (e.g., lower Yakima valley), rust mites may not be numerous, and these areas tend to be more vulnerable to spider mite problems. Drip irrigation, unlike overtree or undertree, does nothing to modify hot, dry conditions.
Cultural factors play a role in spider mite population build-up. High populations have been associated with dusty foliage, such as occurs next to dirt roads. Overtree irrigation, in comparison to undertree irrigation, tends to reduce twospotted and McDaniel spider mite populations, either through microclimate modification or through washing mites from the leaves. European red mite, on the other hand, is little affected by overtree irrigation.
Temperature and humidity also play a role. In areas or years where many heat units accumulate, mite populations can produce more generations. High mite populations are often associated with hot, dry weather (even though in laboratory tests these conditions appear to cause higher mortality). For example, field studies with twospotted spider mite in Oregon show that its potential for population growth is lower in years with below normal temperatures. If the weather is rainy as well as cooler, this reduces survival of mites and will further suppress populations.
The value of the delayed-dormant oil application (at about half-inch green tissue) aimed at overwintering eggs of European red mite has been proven consistently under Washington conditions. Eggs are most vulnerable just prior to hatching, thus true dormant sprays will not be as effective. Usually European red mite populations are prevented from building up through May and June because of the delayed-dormant oil spray, although by itself it does not provide season long control. It does, however, suppress European red mite populations long enough for predators to become established in the trees. In the past, the addition of an organophosphate insecticide has enhanced the efficacy of the oil, but currently most of the effect comes from the oil alone. Mites are less likely to develop resistance to oil than to other insecticides. No report of resistance to oil has yet been documented.
There is evidence on pear that delayed-dormant or pre-pink sprays suppress the overwintering females of twospotted mites, but this suppression does not prevent build-up later in the season. Overwintering females are physiologically and morphologically different than the summer form, and chemical control of this form is more difficult. Specific miticides should be applied after the females lose their orange coloration and before these forms are produced again in the fall.
Effective specific miticides that are registered for use on tree fruits have grown fewer in recent years. In the past, most major groups of insecticides (e.g., the organophosphates) were initially miticidal, but resistance developed within a few years of commercial use. Similarly, most of the specific miticides were ineffective within a few years. The organotins had a relatively long field life, but resistance to these materials is now widespread. Ovicidal miticides have been notorious for their short field life in the past, and experience from other parts of the world indicates this is an ever present danger with the newer ovicides.
An important consideration in choosing a miticide, besides efficacy, is how selective it is toward beneficial arthropods, especially predatory mites. Use of a selective miticide greatly enhances the chance of establishing biological control, which should be the ultimate aim of any mite control action. Even a fairly selective miticide, if sprayed too often or on a prophylactic basis, will deplete the predator population and increase the chance of resistance in the spider mite population. A second consideration is the toxicity of the material to rust mites. Materials with low or moderate toxicity to rust mites will also enhance the chance of establishing or maintaining biological control.
Caution must be exercised not only in the choice of a miticide but also in the choice of insecticides and fungicides. A number of these materials are highly toxic to predatory mites. Two classes notorious for this are the pyrethroids and the carbamates, although not all materials within a given class are equally toxic. Repeated applications of a material only moderately toxic to predators (e.g., organophosphates) may eventually reduce the predator population below an effective level. In the dry areas of the Pacific Northwest, fungicide use is minimal, and this level of use does not appear to present a problem. However, in areas where apple scab is a chronic and serious problem, the selectivity of the fungicide used should be taken into consideration.
Apple
Before specific miticides are applied, the decision must be made as to whether control is warranted. Factors that need to be considered are:
- The level of direct pests and the pesticides used against them
- Damage incurred by other species of foliage feeders
- Tree age and vigor
- Type of irrigation system
- Crop load
- Weather
Although an all inclusive threshold is impossible to quantify, mature, vigorous trees with a light or moderate crop load can withstand a considerable amount of foliar damage without any measurable economic loss. Heavier crop loads require an efficient leaf canopy to mature the fruit properly, whereas if the leaf-to-fruit ratio is high, foliar damage may not be as critical. If the trees are water stressed by extremely hot, dry, or windy weather, a sandy or rocky soil that has poor water retention qualities, or inadequate irrigation, then the effects of mite damage will be exacerbated. If no predators are detected during successive samples and mite populations continue to rise, then miticides may be the only recourse. However, if predator populations are building, but more slowly than the pest mites, a miticide (the most selective one available) may be considered. If the use of a nonselective pesticide is required for another pest, then the chances for biological control are diminished and a miticide is more likely to be needed. In the long run, it may be better to incur some damage from mites initially than to run the risk of reverting to a miticide-based program.
Pear
The heavy use of nonselective programs in pears for pear psylla (pyrethroids, neonicotinyls) has dictated an essentially miticide-based program for mite control. Even before the introduction of pyrethroids, the intensive spray program for psylla was very detrimental to predators. This, coupled with pear foliage’s inherently low tolerance to mite damage, has prevented biological control from playing a major role. The alternate prey component of the system, rust mites, is present on pear but can cause fruit russeting. Thus, the tolerance for rust mite damage is less on pear than on apple (with the exception of Bosc, a naturally russeted pear cultivar), and predatory mites are less able to maintain themselves. The replacement of a harsh, nonselective chemical program for pear psylla with softer materials would allow a greater chance for biological control, although it may never approach the success of integrated mite control on apples. An additional consideration on pear is the effect of orchard floor management on mite populations, especially twospotted spider mite. This species can build to high levels on weed hosts, especially bindweed, beneath the trees. When this food resource is depleted or killed with herbicides, mites may be driven up into the trees in large numbers. A survey of the weed composition and its mite population will indicate whether this is a potential problem.
One strategy is to add a miticide labeled for this use to the herbicide. Preferably, to minimize selection for resistance, the miticide should be of a different chemical class than the one used for the tree canopy. A second strategy is to open the lower nozzles on the airblast sprayer when applying a miticide to the canopy in order to cover the orchard floor. The drawback to this strategy is that development of resistance to the orchard miticide may be hastened.
Success story
Few success stories in the history of tree fruit IPM are more striking than that of integrated control of mites in apple. From the uncontrollable outbreak conditions of the early 1960s, treatable infestations dropped to about 10% of the acreage in the late 1980s and early 1990s. The incidence of mite outbreaks is currently on teh rise, likely linked to the transition away from an organophosphate-bsed insecticide program for codling moth. The newer codling moth materials have are, in general, less acutely toxic to mammals and beneficial insects; however, they appear to be disruptive to integrated mite control.
The basic components of integrated mite control, however, remain unchanged:
- Use of a delayed dormant oil against over-wintering European red mite eggs.
- Maintenance of a moderate rust mite population in the orchard as a food supply for mite predators.
- Use of selective pesticides to protect predators.
In addition, where dust on the foliage has been a problem, reducing it has been very helpful to integrated control. Because our pest complex and our pesticide options change over time, maintaining these practices will present an ongoing challenge.
Re-establishing Typhs
In cases where nonselective pesticide use has destroyed T. occidentalis populations, it may be possible to re-establish them more quickly by seeding the orchard to hasten recolonization. Predators may be reintroduced by taking shoots from an orchard with rust mites and T. occidentalis, and placing them in the affected orchard. Because the movement of T. occidentalis between trees is limited, at least one Typh-infested shoot per tree should be used. If a source orchard is not available, T. occidentalis can be purchased from commercial suppliers of beneficial insects. Strains have been selected in the field and laboratory for tolerance to commonly used orchard pesticides, so make sure that the strain is appropriate for the area of introduction.
^top
Brown Mites
by Elizabeth H. Beers, Published online December 2007
Bryobia rubrioculus (Scheuten) (Acari: Tetranychidae)

This mite species was a well-known pest for most of time tree fruits have been grown in the Pacific Northwest, but has become quite uncommon in the past few decades. A few Washington apple orchards with recurring populations of brown mite were noted in the early 2000s, and it is currently listed as a pest of pear in California. Both the decline and return of brown mite as a pest are likely due to shifts in orchard pesticide programs, but the exact causes are unknown.
In older literature, there is frequent mention of the clover mite, Bryobia praetiosa occurring on tree fruits. This used to be considered a species complex with variations in life history and host plants. B. rubrioculus has been considered a biotype of the complex, which specializes on fruit trees, but work in the mid-1950s declared the forms on tree fruits a separate species (B. arborea a synonym for B. rubrioculus).
Hosts
Hosts include all deciduous fruit trees and almonds.
Life stages
Egg
The overwintering eggs are slightly larger than those of the European red mite, but about the same color (dark brick red). Eggs are laid singly.
Immatures
The larva resembles the larva of European red mite to a large degree (reddish color). At this stage, the long front legs which characterize later stages are not apparent.The protonymphs begin to take on a more distinctive appearance, but still lack the long front legs. The deutonymph (final immature stage) resembles the adult in body shape and color, with long front legs
Adult
The adult female has a characteristic appearance that makes it readily distinguishable from the other common tree fruit tetranychids.The body color is a dull reddish brown with dark orangeish markings, and somewhat flattened. The integument has a striated appearance, with the striations running horizontally across the dorsum. The front legs are very long, over twice the length of the other legs, and extend forward from the body. The eyes are distinct red widely spaced on the cephalothorax.
Life history

This species overwinters as a diapausing egg, much the same as European red mite; however, the eggs of brown mite hatch up to several weeks earlier than those of European red mite (late delayed dormant). Egg hatch continues through April, peaking at pink. Newly hatched forms feed on buds, and later on leaves. Unlike other tree fruit spider mites, brown mites do not spin webs, and they often leave the leaf surface and congregate on twigs, sometimes molting there. Their feeding is thought to be mostly nocturnal. Summer (non-diapausing) eggs are laid on leaves, twigs and spurs. Females live for 2-3 weeks, and lay about 30 eggs. The spring generation immatures molt primarily on the twigs (>90%), while the first summer generation immatures molt primarily on the leaves. There are three to four generations per year. A summer generation begins in July and females of this generation lay aestivating eggs; by late summer the population is composed primarily of the egg form. These eggs remain in diapause through the winter.
Damage
Damage is similar to that caused by other spider mites. All motile stages feed on the leaves and remove chlorophyll. Leaves become stippled from feeding marks, and may become pale or bronzed; however, defoliation is less likely with this species.
Monitoring
Monitoring the first generation must take into account this species habits of congregating on the twigs, thus early samples should be consist of buds, and later leaves and twigs. Sampling later generations can be done with leaf samples using the same method as for other tetranychid species.
Biological control
Brown mite has a number of natural enemies, including predaceous mites, lady beetles, anthocorids, and predatory mirids. However, the relative impact of biological control agents is little studied in Pacific Northwest orchards, and they not considered to be very effective.
Management
There is no current research on this pest, however, older literature suggests that pink sprays are most effective. Specific miticides which are effective for control of other spider mites should also be effective on brown mite.
^top
Brown Mite Gallery
References
Anderson, N. H., and C. V. G. Morgan. 1958. Life-histories and habits of the clover mite, Bryobia praetiosa Koch, and the brown mite, B. arborea M. & A., in British Columbia Can. Entomol. 90: 23-42.
Elkins, R. B., R. A. Van Steenwyk, L. G. Varela, C. Pickel, D. Gubler, and C. L. Elmore. 2002. UC IPM pest management guidelines: Pear. University of California Statewide Integrated Pest Management Program.
Jeppson, L. R., H. H. Keiffer, and E. W. Baker. 1975. Mites injurious to economic plants. University of California Press, Berkeley, CA.
Madsen, H. F., and C. V. G. Morgan. 1970. Pome fruit pests and their control. Ann. Rev. Entomol. 15: 295-320.
Madsen, H. F., and J. C. Arrand. 1966. The recognition and biology of orchard insects and mites in British Columbia. Publ. 66-2, British Columbia Department of Agriculture.
Newcomer, E. J. 1966. Insect pests of deciduous fruits in the west. Handbook 306, United States Department of Agriculture, Washington, DC.