Written by: Tianna DuPont, David Granatstein, and Bernardita Sallato, WSU Extension. Updated February 2020.
Orchard soil health, or soil quality, is the capacity of soil to support productive trees over time without negatively affecting the surrounding environment. Soil health is influenced by interacting biological, physical, and chemical properties of soil. Active soil biological communities mineralize nitrogen, create soil structure, and compete with plant pathogens. Physical properties of soil determine its ability to store and release nutrients; accommodate water entry, storage, and movement; provide sufficient oxygen for roots and microbes; and moderate environmental stress. Chemical aspects of soil health include nutrient presence and availability, pH, cation exchange capacity (CEC), salinity, and the presence of any contaminants, such as heavy metals or persistent pesticide residues.
By increasing our understanding of the biological and physical as well as chemical properties of soil we may be able to increase root health, moderate nutrient and water stress, and increase the yield potential of our orchards.
Functions Provided by Healthy Soil
Root Health
Root growth and development are controlled by multiple factors including soil microorganisms, oxygen, soil temperature, water availability, nutrients, and management practices (Atkinson and Wilson 1980).
Large active microbial communities are important to reduce pest and pathogen pressure and maintain root health (Figure 1). They do this both by competing for resources and by directly attacking pests and pathogens (van Os and van Ginkel 2001; Garbeva et al. 2004). For example, some groups of microbes produce antibiotics (Streptomyces, and numerous strains of Micromonespora and Nocardia). Some Pseudomonas strains produce antibiosis reducing pathogen pressure. Other microbes such as the fungi Trichoderma, actively parasitize plant pathogens. Orchard soils higher in Trichoderma, as well as Streptomyces and Pseudomonas bacteria, have been found to be more suppressive to the pathogens involved in replant disease than soils with lower levels of beneficials (Weerakoon et al. 2012).

Healthy, high-quality soil has:
- Good soil tilth
- Sufficient depth
- Sufficient, but not excessive, nutrient supply
- Small population of plant pathogens and insect pests
- Good soil drainage
- Large population of beneficial organisms
- Low weed pressure
- No chemicals or toxins that may harm the crop
- Resilience to degradation and unfavorable conditions
Excerpt from: Soil Health Training Manual (Gugino et. al, 2007)

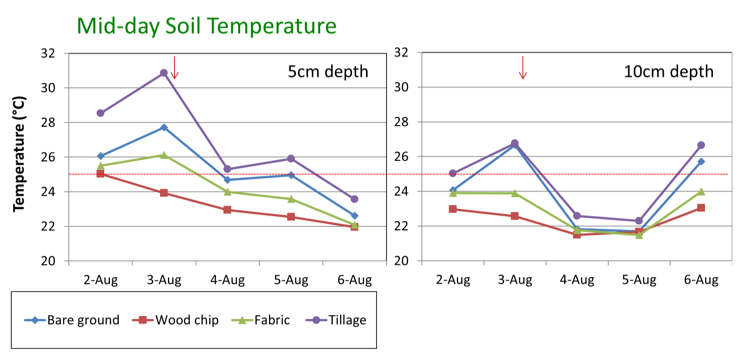
Roots thrive in environments with sufficient oxygen and optimal temperatures. Researchers have proposed that the optimal temperatures for apple roots are 64 to 77oF. Soil temperatures above 86oF seem to be deleterious to roots (Gur et al. 1972). Tolerance to soil temperatures varies by genetics. For example, in one study roots from an M.9 rootstock died at 77oF. At high temperatures, roots matured fast, browned, sloughed, and were infected by pathogens (Nelson and Tukey 1956). In another study, soil temperature less than 59oF delayed bud break, and resulted in fewer flower clusters on ‘Braeburn’/’M.9′ (Greer et al. 2006). In cherries, adequate temperatures for ‘Gisela’ rootstocks in Chile were between 57oF to 83oF, below which there was no growth. Optimum temperatures for root growth, however, could change in different growing regions (Bonomelli et al. 2012).
Soil management may be important for reducing surface soil temperature and root stress in Washington summers. For example, in a recent Washington study, tree row strips with bare ground or black weed fabric had above optimum temperatures (above 77oF, 25oC) at 2.5 inches (5 cm) (Figure 2). In contrast, soils with wood chip mulch had temperatures below the 77oF (25oC) stress point.
Nutrient Availability

Soil organic matter acts like a bank for soil nutrients. Think of each of the negative charges on an organic matter particle like a parking spot for a nutrient ion. Cationic nutrients, such as magnesium (Mg2+), are parked and ready to be knocked out into the soil solution where plants can access them. Root exudates from plants help ‘knock’ nutrients into solution by trading these nutrients (such as magnesium) with hydrogen ions. The higher the cation exchange capacity (CEC), the more of these parking spaces for nutrients are present in the soil. More nutrients can then be held instead of being washed away into deep soil layers where plants can’t access them. In coarse textured soils, like sand, organic matter is the dominant source of CEC (Figure 3).
Organic matter not only banks nutrients but also supplies nitrogen through mineralization. Organic matter contains about five percent nitrogen, and two to four percent of this is mineralized every year. For example, a soil with three percent organic matter can make available sixty pounds of nitrogen per acre every year (as long as soil organic matter is maintained).
Soil biota are another important regulator of nutrient availability. Bacterial and fungal feeding nematodes, protozoa, and microarthropods significantly impact soil nutrient retention and release. When biota eat bacteria, fungi or each other, they excrete nitrogen in the plant available form, such as ammonia (NH4-N). For example, in one study of grassland soils, nematodes mineralized as much as eight to twenty-two percent of available nitrogen (Elliott et al. 1988), and twenty-eight pounds of nitrogen per acre per year in another study (Culman et al. 2010). With mesofauna, such as mites and springtails, feeding on microbes excrete nitrogen-rich waste. This mineralization can contribute up to thirty percent of soil-supplied nitrogen (Deruiter et al. 1993; Griffiths 1994), and increasing plant uptake up to fifty percent (Laakso et al. 2000). Additionally, mycorrhizae act to mobilize phosphorus and other nutrients making them more available to plants (Jansa 2011).
Water Availability

Trees need sufficient but not excess plant-available soil water to maintain growth, yield, fruit size, and fruit quality (Naor et al. 1995). Calcium related disorders, such as bitter pit, are more common either with insufficient or excess water (de Freitas and Mitcham, 2012). Excess water can also lead to quality problems, including cork spot (Brun et al. 1985).
Excess water from poor drainage, or waterlogging caused by continual overhead cooling, can encourage pathogens to proliferate, lead to anaerobic conditions that disrupt root function, and can cause nutrient loss through denitrification or leaching. Soils with low bulk density, high structure, and high porosity have better drainage (Figure 4).
Soil structure and quality moderate plant water availability. Soil organic matter acts like a sponge holding soil water and releasing it to plants slowly over time (e.g., Figure 3). When soil organic matter increases, available water capacity can increase. For example, in one study, water capacity increased from 5 to 25% by volume as organic matter increased from one-half to three percent (Hudson 1994). The increase in available water with organic matter is greatest for sandy soils and may be negligible in clay soil types (Minasny and McBratney 2018). Other soil quality indicators, such as compaction, greatly impact root available water (Mathers 2004). Surface mulches help reduce evaporative water loss from soil and can reduce periods of water stress as well as overall water use (Granatstein and Mullinix 2008).
Relationship between Soil Quality and Yield
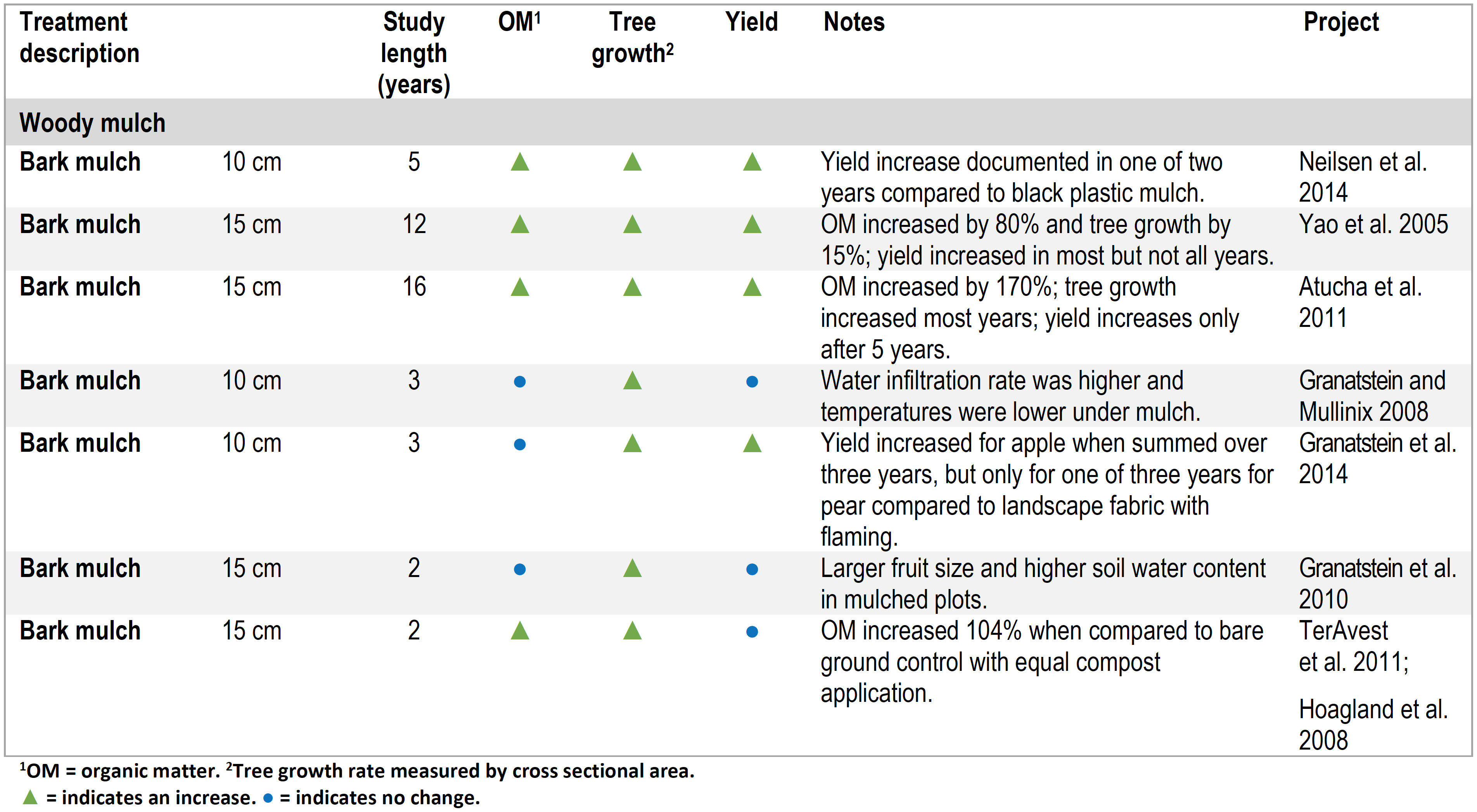
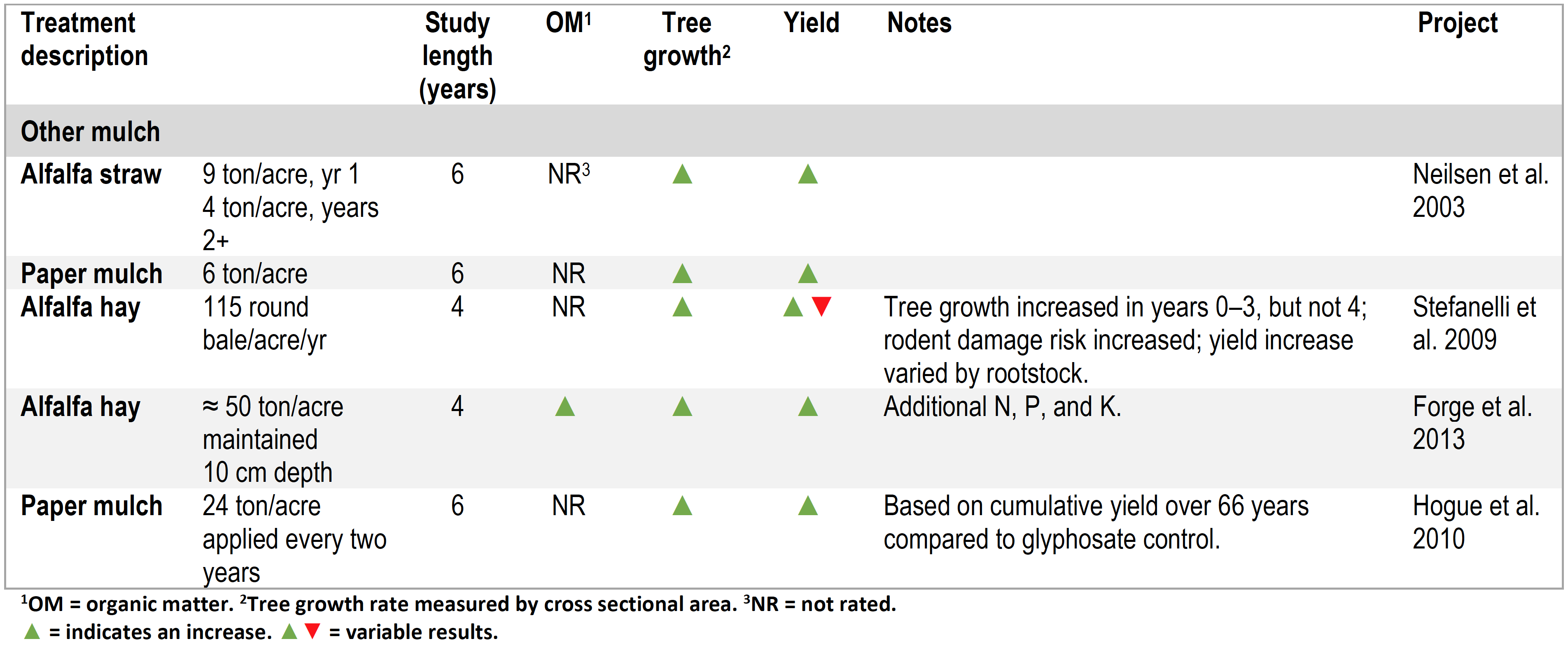
Healthy soils have been shown to support larger, more stable, yields (Figure 5) (Pimentel et al. 2005; Chivenge et al. 2011). However, much more work is needed to investigate this relationship in orchard systems. This section reviews a number of orchard floor management studies in order to share current knowledge about which soil health management practices may positively impact the grower’s bottom line (Tables 1 to 4). As available studies measured only a limited number of soil quality indicators, this section focuses on organic matter, the only indicator commonly measured to date.
Yield and or tree growth (or both) increased in all studies where soil organic matter levels increased (seven in total of thirteen studies where organic matter was added and treatment effects on yield and organic matter documented) (Tables 1 to 3) (Neilsen et al. 2014; Yao et al. 2005; Atucha et al. 2011; Sanchez et al. 2003; Reeve et al. 2017; TerAvest et al. 2011; Hoagland et al. 2008; Forge et al. 2013). In three treatments where neither yield nor growth increased, soil organic matter increases had not been achieved (Neilsen et al. 2014, Sanchez et al. 2003).

Growth and yield increases were documented in other studies. However, none reported organic matter or other soil quality indicators, and, as such, direct correlation between yield increase and soil quality is not possible (Hogue et al. 2010; Stefanelli et al. 2009).
Table 1. Summary of orchard floor management studies with compost, manure, or mown alleyway organic matter additions.
Studies with Wood Chip Mulch
Soil quality parameters such as soil water holding capacity, nutrient cycling, and biological activity can impact tree growth and yield even when soil organic matter levels have not (or not yet) changed. This is apparent in several studies with wood chip-based mulches where tree growth and yield improved even when soil organic matter did not measurably change (Table 2). Mulch can influence soil temperature, water status, nutrients, and biological activity. For example, chip mulch improved tree growth but not fruit yield when compared with the bare ground control in a four-year study on mature ‘Red Delicious’/’M.26’ apple trees near Wenatchee, WA (Table 2). The mulch did not increase soil organic matter but did enhance potential biological N mineralization (Granatstein and Mullinix 2008). In a related study in the same orchard, mulching increased tree growth and fruit size, but not yield or active carbon after three years (Granatstein et al. 2010). In another study, mulching led to greater tree growth and fruit yield compared with tillage in a mature, organic ‘Gala’/’M.26’ orchard in central Washington (Granatstein et al. 2014). In a newly planted apple orchard, mulching improved tree growth and yield over three years. Total soil C and N in this orchard increased slowly with treatment differences only becoming apparent in year three (Hoagland et al. 2008; TerAvest et al. 2011).
Table 2. Summary of orchard floor management studies using wood chip mulch.
Table 3. Summary of orchard floor management studies using non-woody mulches.
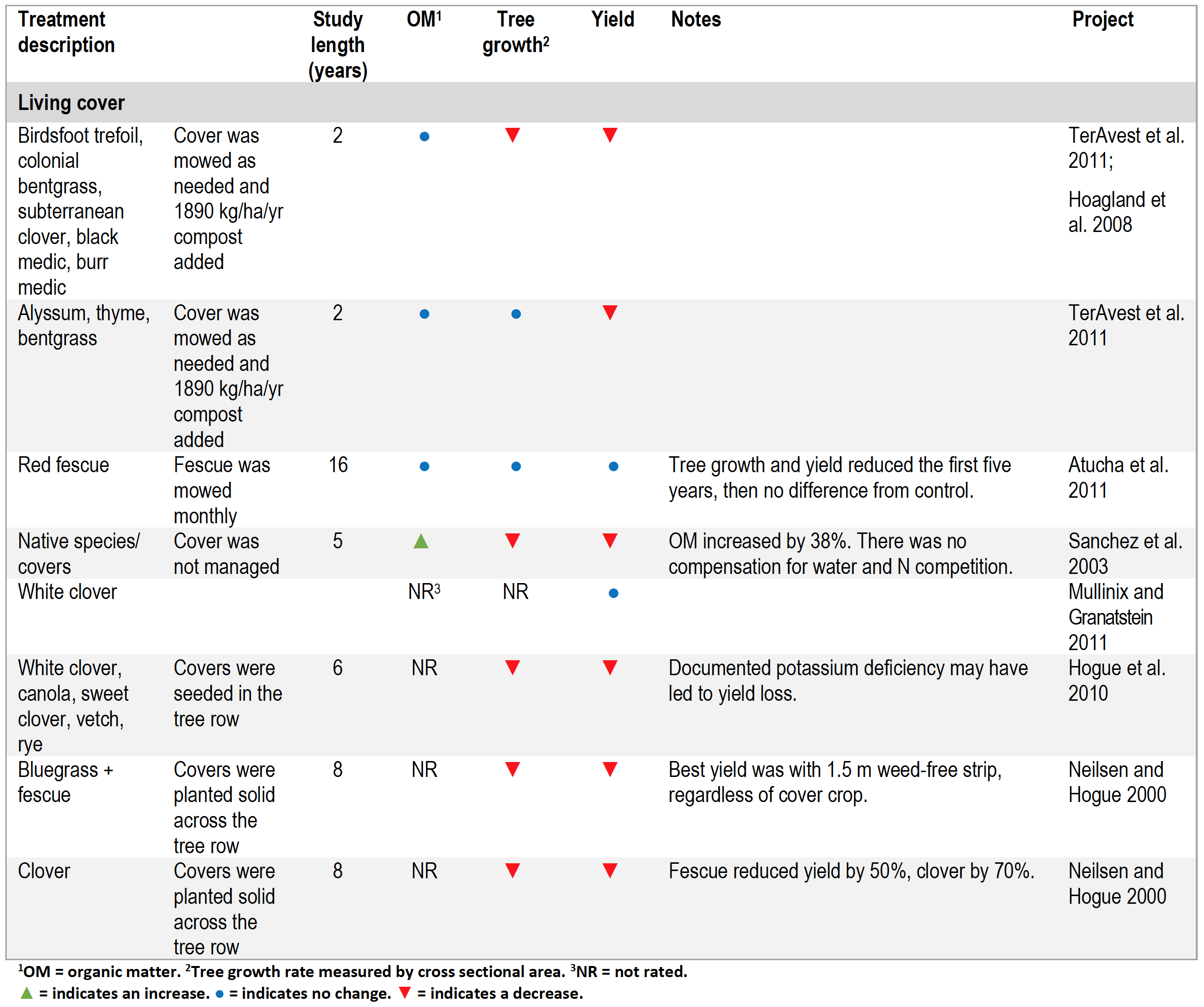
Studies with Living Mulches
In most living mulch studies where the grass, legume or forb was planted in the tree row as well as the drive-row, tree growth and yield were lower compared to the bare ground controls (Table 4). The one notable exception was a sixteen-year study in New York in which fescue grown in tree rows was mowed monthly. While tree growth was reduced during the first three years after a living cover was implemented, over time tree growth and yield were as high as the control, suggesting that trees were able to adapt to the grass thatch by developing deeper root structures able to access water and nutrients below grass feeder roots (Atucha et al. 2011).
Table 4. Summary of living cover studies.
Soil Health Indicators
Current work is investigating which soil health indicators are most important for orchards. Several potentially important indicators include soil compaction, water holding capacity, water infiltration, organic matter, soil biota, soil pH, and soil nutrients.
Soil Compaction

Productive soil has sufficient pore space filled with air and water (about 50% soil particles and 50% pore space) to keep roots healthy (Brady and Weil 1996). Using heavy equipment on soil that is too wet can push soil particles closer together, physically compacting soil, leaving smaller pores with less air and water for plants (Duiker, 2004). When soil is highly compacted, roots can no longer penetrate, restricting root growth and access to water and nutrients in these soil layers (Figure 6). Compaction not only affects root growth, it also reduces the volume of air-filled pores and, consequently, oxygen in the soil which can stress plant roots.
Measuring soil compaction: A penetrometer measures the force required to press a metal rod into the soil. Compaction over 300 pounds per square inch (psi) can restrict root growth.
Managing soil compaction: Reducing traffic, mechanically loosening with a deep ripping tool during orchard renovation, planting cover crops with strong taproots which penetrate compaction layers, and additions of organic matter can reduce compaction. Keep in mind that deep ripping may only mitigate compaction for one to two years if not combined with organic matter additions and reductions in traffic.
Water-Holding Capacity
A soil’s ability to hold water and supply it slowly over time is critical to limiting plant stress. Water-holding capacity is the amount of water the soil can hold between field capacity and the permanent wilting point. A soil that has been fully saturated by a heavy rain or long irrigation and allowed to drain but not dry is at field capacity. The permanent wilting point is defined as the soil moisture level at which a wilted plant cannot recover even after twelve hours in a remoistened soil. Soils with higher clay or organic matter levels have higher water-holding capacity than sandy soils.
How to Improve Soil’s Water Holding Capacity: Applications of manure, compost, and cover crops add organic matter to soils and can slowly increase soil water holding capacity.
Measuring Available Water Capacity: Available water capacity is determined by measuring water content at field capacity and at the permanent wilting point in the lab and calculating the difference. This is done by measuring soil water content at different pressures to simulate field capacity (small negative pressure) and permanent wilting capacity (large negative pressure). The amount of water held between the extremes is the available water (Gugino et al. 2007).
Water Infiltration

Movement of water into and through the soil is critical to ensure that water reaches plant roots and does not wash off the soil surface or pond (creating anaerobic areas where plant roots cannot breathe). Good water movement also helps ensure that water soluble nutrients move through the soil into the rooting zone for uptake by trees. Infiltration rate is largely determined by the size of the pores at the soil surface. Water infiltrates faster into soil with larger pore spaces than smaller pore spaces. While managers cannot change the texture of the soil at a given site, soil organic matter additions can increase pore size and thereby improve soil structure and water movement. It is important to minimize traffic in wet soils which may compress soils and reduce infiltration. Roots of cover crops in drive rows and earthworm holes can also create macropores which increase infiltration. In sandy soils common in Washington orchards, infiltration and drainage can also be excessively high.
Measuring water infiltration: Infiltration rate is a measurement of how much water can infiltrate into the soil surface in a given amount of time. A simple way to measure infiltration rate is to insert a plastic or metal ring into the soil surface, add a given amount of water, and measure how long it takes the water to infiltrate into the soil (Figure 7).
Organic Matter
Soil organic matter (SOM) is the “living, dead and very dead” fractions of the soil, including fresh plant and animal residue, living and dead microorganisms, and well-decomposed residue (Magdoff and van Es 2009). Crop residues, manure, compost, and cover crop residues all contribute to SOM in agricultural systems.
Why Is Soil Organic Matter So Important? While the percent of SOM in soil is small (averaging one to six percent in agricultural soils), it influences many properties and processes (Figure 8).
The living fraction of soil organic matter includes bacteria, fungi, protozoa, earthworms, and tiny insects. Soil biota composes about 15% of total soil organic matter, weighing between 2,000 and 30,000 pounds per acre (Gugino et al. 2007; Brady and Weil 1996). This living fraction of the soil mineralizes nitrogen, improves soil structure, assists in decomposition, and moderates pathogen and pest pressure.
The “dead” soil organic matter fraction, made up of recently added plant and animal residue, is easily decomposed. Most organic matter decays quickly to carbon dioxide, water, and minerals. Fresh soil organic matter is important because it provides the food and, thus, energy (i.e., via respiration) for soil organisms and mineral nutrients for crops. Soils with more recently added carbon (often called “active”), have more carbohydrates and sugars readily available to soil microbes. Active carbon also often serves as an early indicator of changes in total organic matter. These soils generally have larger and more stable soil food webs.
Another fraction of soil organic matter is protected from decomposition and can make up about 70% of the total SOM in agricultural soils.
Measuring organic matter: Total organic matter is measured by combustion. Active carbon can be measured several ways, including potassium permanganate oxidation and particulate organic matter measured by sieving (Gugino et al. 2007).
Root Pathogen Pressure

Pathogen pressure is an important indicator of plant health. High pathogen pressure indicates that disease‐causing organisms are present and impacting roots. Lower pressure indicates either that few pathogens are present, or that the rest of the microbial community is able to prevent them from successfully colonizing the roots. For tree fruits, it can be measured by an apple root bioassay where apple seedlings are grown in pasteurized and non-pasteurized soil for six weeks and subsequent growth measured (Figure 9).
Soil Biota
The soil is alive. In one cubic foot of soil there may be 2 trillion bacteria, 3 miles of fungal hyphae, 4 million protozoa, 30 thousand nematodes and hundreds of microarthropods (Kounang and Pimentel 1998). Large, active biological communities are essential for healthy plants.
Earthworms and insects act as “ecosystem engineers” mixing soil and creating soil structure. Tiny insects tear and shred leaves and other plant material into small pieces easily decomposed by bacteria and fungi. Bacteria and fungi help create soil structure by holding soil particles together into aggregates with long fungal hyphae and sticky substances produced by bacteria. Microscopic worms, called nematodes, as well as protozoa and tiny springtails consume these bacteria and fungi, excreting nitrogen available to plants.
Measuring soil biological activity: There are many ways to measure soil biological activity. Soil respiration indicates microbial abundance and quantifies the metabolic activity of the microbial community at a point in time by capturing and quantifying carbon dioxide produced by respiration. Soil enzymes can be measured to indicator biological activity. New genetic tests can create a DNA “fingerprint” of the soil and also indicate which organisms are most active.
Increasing biological activity: Compost, manure, and crop residues all provide organic matter and food for the soil food web. Materials high in lignin-based carbon tend to encourage fungi which can break down tough-to-digest material with enzymes such as chitinase. Materials high in nitrogen or with small particle size tend to encourage bacteria.
See Soil Biota in Orchards (DuPont 2019) for additional information.
Microbial Available Nitrogen
Nitrogen stored in soil organic matter is made available slowly over time by microbial activity. The quantity of nitrogen stored in soil organic matter and the activity of biological communities determine plant nitrogen availability.
Measuring microbial available nitrogen: Several tests measure microbially available nitrogen. Potentially Available N is determined by potassium chloride reactions. (Clune et al. 2016), and potential N mineralization uses an incubated sample (e.g., 7-day anaerobic incubation). Soil protein analysis measures the fraction of the soil organic matter present as proteins or protein-like substances. Protein content, as organically bound N, influences the ability of the soil to make N available by mineralization. It is measured by citrate buffer under high temperature and pressure.
Soil pH
pH is considered a master property of soil in that it influences many chemical and biological processes. Use of acidifying nitrogen fertilizers can lower pH over time. When fertilizer is focused on the tree row, dramatic differences in pH between the tree row and alley may appear. For example, in five Okanagan Valley, BC orchards, soil pH in the tree row was 3.9–4.4 (undesirable for tree growth) versus 5.4–6.7 in the alley (Ross et al. 1985). Low pH increased exchangeable aluminum and the exchangeable cation ratios. Liming is the typical solution to this problem. High pH is common in central Washington orchard soils. Sulfur applications can bring pH back into the 6.0 to 6.5 range optimum for tree fruit.
Soil Nutrients
Macronutriens like nitrogen, phosphorus, and potassium, as well as micronutrients are essential to plant growth and soil health. For an in-depth discussion see Tree Fruit Soil Fertility and Plant Nutrition in Cropping Orchards in Central Washington (Sallato et al. 2019).
Improving Soil Health in Orchards
Significant research is needed to determine the best practices for improving soil health in orchards. The following are basic principles based on current knowledge.

Feed the soil
An important step for improving soil health in orchards is to boost the “carbon cycle” in the tree root zone by recycling pruned material and leaves, mowing and blowing grass strips into the tree row (mow-and-blow), applying organic mulches, applying compost and other organic matter sources (Figure 10). A steady application of carbon inputs over time improves a soil’s ability to hold and cycle water and nutrients, combat pathogen invaders, and provide a beneficial root environment. Practices such as mulching and mow-and-blow of the alley vegetation onto the tree row can provide an insulating mulch, increase the water holding capacity, and provide carbon inputs that stimulate soil health.
Consider woody mulches
Woody mulches have most consistently improved tree growth and yield likely due to their ability to moderate soil water and temperature stress to trees (Figure 11). While importing woody chipped material is expensive, consider options like flail mowers which are able to chip prunings in place, saving labor on removing material and providing on-site carbon sources. Move the flailed material to the tree row with a brush rake or other tool to put it where it will do the most good.

Minimize disturbance
In general, orchards excel at minimizing soil disturbance. Most orchards have tillage at a single point during their life – at the time of planting. This may involve deep ripping to help alleviate compaction (a plus for soil quality), rototilling the tree row to incorporate fertilizer or amendments, and preparing a seedbed in the alley for the cover crop. Minimal disturbance helps maintain soil structure and fungal networks and minimizes oxidation of organic matter. It also keeps residue on the soil surface to protect against soil erosion. In organic orchards, tillage may be used several times a year for weed control in the tree row. Erosion risk is minimal due to vegetated alleys. Some growers use tillage during the years of tree establishment and then let vegetation move into the tree row which they control with mowing, flaming, or organic herbicides.
Living Roots
Most modern orchards have perennial vegetation in the drive alley, typically a grass mixture. Thus, half or more of the soil surface has permanent cover year–round, which is excellent for erosion control, soil structure, water infiltration, and carbon supply to soil via root exudates, dying roots, and decomposing clippings. Tree rows kept bare by repeated herbicide treatment do show loss of structure and water infiltration in some cases.
Don’t forget pH and nutrients
Soil health involves the chemical as well as physical and biological properties of soil. While we expand our consideration of soil health to include biological and physical properties, we cannot forget to monitor and manage major and micronutrients. For an in-depth discussion see Tree Fruit Soil Fertility and Plant Nutrition in Cropping Orchards in Central Washington (Sallato et al. 2019).
Summary
Water and nutrient availability and healthy roots are essential to optimize fruit quality and productivity. A greater understanding of the biological and physical as well as chemical properties of soil can improve managers’ ability to improve these essential soil functions in orchards.
Acknowledgments
Previously published in part DuPont, S. T., 2012. Soil Quality Information. Penn State Extension EE0040.
Commercial Soil Health Testing
- A&L Western Laboratories conduct nutrient testing and nematode analysis.
- AgSource Laboratories Umatilla, OR. 509-727-6058 offers two soil health packages which measure soil nutrients as well as organic matter and microbial respiration.
- Best-Test Analytical Services, LLC Moses Lake, WA. 509-766-7701 provides soil nutrient and organic matter testing.
- Brookside Laboratories, Inc. New Knoxville, OH. 419-753-2448 provides a soil health test that includes microbial respiration by Solvita measurement, water extractable carbon and nitrogen, and available organic nitrogen as well as standard nutrient analysis.
- Cornell offers biological, physical and chemical property of soil testing including soil pH, organic matter, modified morgan extractable P, K, micronutrients, soil texture, active carbon, wet aggregate stability, soil respiration, extractable protein, available water capacity, soluble salts, root health bioassay, and potentially mineralizable nitrogen. Note: Olsen extraction (P and K) is recommended in Central Washington.
- Dellavalle Laboratory, Inc. Fresno, CA 800 228-9896 provides soil nutrient and nematode analysis.
- Northwest Agricultural Consultants Inc. Kennewick, WA 509-783-7450 offers soil nutrient analysis, Haney test, organic matter, and nematode analysis.
- Oregon State University offers biological, physical and chemical property of soil testing including soil pH, organic matter, nutrient analysis, micronutrients, soil texture, active carbon, wet aggregate stability, soil respiration, extractable protein, available water capacity, soluble salts, and potentially mineralizable nitrogen.
- Soiltest Farm Consultants Moses Lake, WA. 509-765-1622 offer nutrient and nematode analysis as well as soil respiration and active carbon.
Orchard Chipping Services
Ziemke’s Orchard Removal. 1670 Smith Rd Zillah WA 98953. (509) 379-1166 Is able to grind with trellis wire and remove metal.
Somers Orchard Removal. (509) 797-3514
Ironsides Custom Grinding. PO Box 8 Waitsburg WA 99361. (509) 316-6109
Additional Resources
- Doran, J. W., D.C. Coleman, and D.F. Bezdicek (eds). 1994. Defining Soil Quality for a Sustainable Environment. Madison, WI: Soil Science Society of America. doi: http://10.2136/sssaspecpub35.frontmatter.
- Duiker, S.W. 2002. Diagnosing Soil Compaction Using a Penetrometer (Soil Compaction Tester). PenState Extension Agronomy Facts 63.
- Magdoff, F., and H. M. van Es. 2009. Building Soils for Better Crops. Beltsville, MD: Sustainable Agriculture Research and Education Program.
References
Atkinson, D. and S.A. Wilson. 1980. The growth and distribution of fruit tree roots: some consequences for nutrient uptake. In Mineral Nutrition in Fruit Trees. Edited by D. Atkinson, J.E Jackson, R.O. Sharples and W.M. Waller. London: Butterworth. pp. 137-150.
Atucha, A., I. A. Merwin, and M. G. Brown. 2011. Long-term effects of four groundcover management systems in an apple orchard. Hortscience 46 (8):1176-1183.
Bonomelli, C., C. Bonilla, E. Acuna, and P. Artacho. 2012. Seasonal pattern of root growth in relation to shoot phenology and soil temperature in sweet cherry trees (Prunus avium): A preliminary study in central Chile. Ciencia e Investigacion Agraria 39 (1):127-136.
Brun, C.A., J.T. Raese, and E.A. Stahly. 1985. Seasonal response of ‘Anjou’ pear trees to different irrigation regimes. II. Mineral composition of fruit and leaves, fruit disorders, and fruit set. J. Am. Soc. Hortic. Sci. 110:835-840.
Chivenge, P., B. Vanlauwe, and J. Six. 2011. Does the combined application of organic and mineral nutrient sources influence maize productivity? A meta-analysis. Plant Soil 342 (1-2):1-30. doi: 10.1007/s11104-010-0626-5.
Culman, S. W., S. T. DuPont, J. D. Glover, D. H. Buckley, G. W. Fick, H. Ferris, and T. E. Crews. 2010. Long-term impacts of high-input annual cropping and unfertilized perennial grass production on soil properties and belowground food webs in Kansas, USA. Agric. Ecosyst. Environ. 137 (1-2):13-24. doi: 10.1016/j.agee.2009.11.008.
de Freitas, S.T., and E.J. Mitcham. 2012. Factors involved in fruit calcium deficiency disorders. In Horticulture Reviews. Edited by J. Janick. Berkely: Wiley. doi: 10.1002/9781118351871.ch3
Deruiter, P. C., J. A. Vanveen, J. C. Moore, L. Brussaard, and H. W. Hunt. 1993. Calculation of nitrogen mineralization in soil food webs. Plant Soil. 157 (2):263-273.
Duiker, S. W. 2004. Effects of soil compaction. Penn State Extension (ed.). University Park: Penn State University.
Elliott, E. T., H. W. Hunt, and D.E. Walter. 1988. Detrital foodweb interactions in North-American grassland ecosystems. Agric. Ecosyst. Environ. 24:41-56.
Forge, T., G. Neilsen, D. Neilsen, E. Hogue, and D. Faubion. 2013. Composted dairy manure and alfalfa hay mulch affect soil ecology and early production of ‘Braeburn’ apple on M.9 rootstock. Hortscience 48 (5):645-651.
Garbeva, P., J. A. van Veen, and J. D. van Elsas. 2004. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 42:243-270. doi: 10.1146/annurev.phyto.42.012604.135455.
Granatstein, D. 1999. Compost effects on apple tree growth. The Compost Connection 1 (10).
Granatstein, D., P. Andrews, and A. Groff. 2014. Productivity, economics, and fruit and soil quality of weed management systems in commercial organic orchards in Washington State, USA. Organic Agriculture 4:197-207.
Granatstein, D., and A. Kukes. 2013. Mow and blow trial, Larson block, WMO, Quincy, WA. Unpublished data. Washington State University.
Granatstein, D., and K. Mullinix. 2008. Mulching options for northwest organic and conventional orchards. Hortscience 43:45-50.
Granatstein, D., M. Wiman, and E. Kirby. 2010. Sustainability trade-offs in organic orchard floor management. Proc. Organic Fruit. Acta Hort. 873.
Greer, D. H., J. N. Wunsche, C. L. Norling, and H. N. Wiggins. 2006. Root-zone temperatures affect phenology of bud break, flower cluster development, shoot extension growth and gas exchange of ‘Braeburn’ (Malus domestica) apple trees. Tree Physiology 26 (1):105-111.
Griffiths, B. S. 1994. Microbial feeding nematodes and protozoa in soil: Their effects on microbial activity and nitrogen mineralization in decomposition hot spots and the rhizosphere. Plant Soil 164 (1):25-33.
Gugino, B.K., O.J. Idowu, R.R. Schindelbeck, H. M. van Es, D.W. Wolf, B.N. Moebius, J.E. Theis, and G.S. Abawi. 2007. Soil Health Training Manual. Ithica: Cornell University.
Gur, A, B Bravdo, and Y Mizrahi. 1972. Physiological responses of apple trees to suboptimal root temperature. Physiol. Plant. 27 (2).
Hoagland, L., L. Carpenter-Boggs, D. Granatstein, M. Mazzola, J. Smith, F. Peryea, and J. P. Reganold. 2008. Orchard floor management effects on nitrogen fertility and soil biological activity in a newly established organic apple orchard. Biol. Fertil. Soils 45 (1):11-18. doi: 10.1007/s00374-008-0304-4.
Hogue, E. J., J. A. Cline, G. Neilsen, and D. Neilsen. 2010. Growth and yield responses to mulches and cover crops under low potassium conditions in drip-irrigated apple orchards on coarse soils. Hortscience 45 (12):1866-1871.
Hudson, B. D. 1994. Soil organic-matter and available water capacity. J. Soil Water Conserv. 49 (2):189-194.
Jansa, J. 2011. Role of mycorrhizal symbioses in phosphorus cycling. In Phosphorus in Action: biological processes in soil phosphorus cycling. E. Bünemann, A. Oberson and E. Frossard (eds.). Soil Biology Vol. 26. New York: Springer.
Kounang, N, and D. Pimentel. 1998. Ecology of soil erosion in ecosystems. Ecosystems 1 (5):416-426.
Laakso, J., H. Setala, and A. Palojarvi. 2000. Influence of decomposer food web structure and nitrogen availability on plant growth. Plant Soil 225 (1-2):153-165.
Minasny, B., and A.B. Mcbratney. 2018. Limited effect of organic matter on soil available water capacity. Eur. J. Soil Sci. 69:39-47.
Mullinix, K., and D. Granatstein. 2011. Potential nitrogen contributions from legumes in Pacific Northwest apple orchards. Intl. J. Fruit Sci. 11 (1):74-87.
Naor, A., I. Klein, and I. Doron. 1995. Stem water potential and apple size. J. Am. Soc. Hortic. Sci. 120 (4):577-582.
Neilsen, G. H., and E. J. Hogue. 2000. Comparison of white clover and mixed sodgrass as orchard floor vegetation. Can. J. Plant Sci. 80 (3):617-622.
Neilsen, G. H., E. J. Hogue, T. Forge, and D. Neilsen. 2003. Surface application of mulches and biosolids affect orchard soil properties after 7 years. Can. J. Soil Sci. 83 (1):131-137.
Neilsen, Gerry, Tom Forge, Denis Angers, Denise Neilsen, and Eugene Hogue. 2014. Suitable orchard floor management strategies in organic apple orchards that augment soil organic matter and maintain tree performance. Plant Soil 378 (1-2):325-335. doi: 10.1007/s11104-014-2034-8.
Nelson, E. H., and H.B. Tukey. 1956. Effects of controlled root temperatures on the growth of East Malling rootstocks in water culture. J. Hortic. Sci. 31 (1):55-63. doi: 10.1080/00221589.1956.11513857
Pimentel, D., P. Hepperly, J. Hanson, D. Douds, and R. Seidel. 2005. Environmental, energetic, and economic comparisons of organic and conventional farming systems. Bioscience 55 (7):573-582. doi: 10.1641/0006-3568(2005)055[0573:eeaeco]2.0.co;2.
Reeve, J.R. , C.M. Culumber, B.L. Black, A. Tebeau, C.V. Ransoma, D. Alstonc, M. Rowleya, and T. Lindstroma. 2017. Establishing peach trees for organic production in Utah and the Intermountain West. Sci. Hortic. 214:242-251.
Ross, G.J., P. B. Hoyt, and D. Neilsen. 1985. Soil chemical and mineralogical changes due to acidification in Okanogan apple orchards. Can. J. Soil Sci. 65:347-355.
Sanchez, J. E., C. E. Edson, G. W. Bird, M. E. Whalon, T. C. Willson, R. R. Harwood, K. Kizilkaya, J. E. Nugent, W. Klein, A. Middleton, T. L. Loudon, D. R. Mutch, and J. Scrimger. 2003. Orchard floor and nitrogen management influences soil and water quality and tart cherry yields. J. Am. Soc. Hortic. Sci. 128 (2):277-284.
Stefanelli, D., R.J. Zoppolo, and R. Perry. 2009. Organic orchard floor management systems for apple effect on rootstock performance in the midwestern United States. Hortscience 44 (2):263-267.
TerAvest, D., J.L. Smith, L. Carpenter-Boggs, D. Granatstein, L. Hoadland, and J. Reganold. 2011. Soil carbon pools, nitrogen supply, and tree performance under several groundcovers and compost rates in newly planted apple orchard. Hortscience 46 (12).
van Os, G. J., and J. H. van Ginkel. 2001. Suppression of Pythium root rot in bulbous Iris in relation to biomass and activity of the soil microflora. Soil Biol. Biochem. 33 (11):1447-1454. doi: 10.1016/s0038-0717(01)00053-0.
Weerakoon, D. M. N., C. L. Reardon, T. C. Paulitz, A. D. Izzo, and M. Mazzola. 2012. Long-term suppression of Pythium abappressorium induced by Brassica juncea seed meal amendment is biologically mediated. Soil Biol. Biochem. 51:44-52. doi: 10.1016/j.soilbio.2012.03.027.
Yao, S., I. A. Merwin, G. W. Bird, G. S. Abawi, and J. E. Thies. 2005. Orchard floor management practices that maintain vegetative or biomass groundcover stimulate soil microbial activity and alter soil microbial community composition. Plant Soil 271 (1-2):377-389. doi: 10.1007/s11104-004-3610-0.